6.7.1
Amino Acids (A2 Only)
Amino Acids
Amino Acids
There are about 20 naturally-occurring amino acids in animal cells.
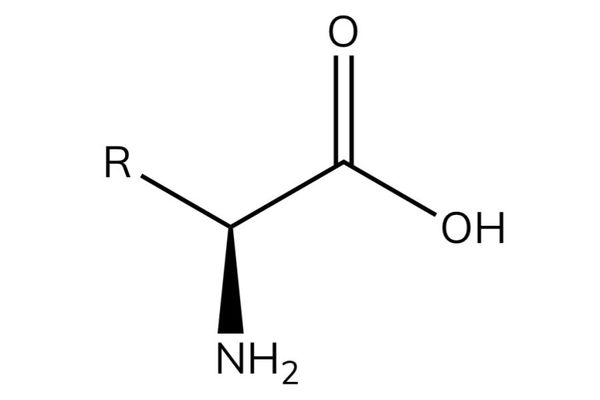

Amino acids
Amino acids
- Amino acids have the general formula RCH(NH2)COOH.
- Amino acids contain an acidic group (carboxylic acid) and a basic group (amine) in the same molecule.
- The -NH2 group is on the carbon next to the -COOH group and so they are known as α-amino acids.


Acid and base behaviour
Acid and base behaviour
- The -COOH group is acidic and can react with bases by donating a proton (H+).
- The -NH2 group is basic and can react with acids by accepting a proton (H+).
- Amino acids are amphoteric because they contain both acidic and basic groups in the same molecule.


Side chain
Side chain
- An amino acid contains an -R group which is a side chain.
- This is different for different amino acids.
- E.g. Alanine has an -R group of -CH3.
The Zwitterion Form
The Zwitterion Form
An amino acid can exist in the zwitterion form.


Zwitterion form
Zwitterion form
- Molecules or ions having separate positively and negatively charged groups are known as zwitterions.
- An internal acid-base reaction in an amino acid causes the nitrogen of the amine group to accept a proton from the carboxylic acid group.


Zwitterion form
Zwitterion form
- The zwitterion makes the amino acid have ionic charges.
- A positive charge is on the protonated nitrogen.
- A negative charge is on the deprotonated oxygen.
- The ionic nature of amino acids gives them higher melting points than expected.


pH on the zwitterion
pH on the zwitterion
- At low pH, the carboxylic acid will not be deprotonated (no charge) and the nitrogen will have accepted a proton (positive charge).
- At high pH, the amine group will not be protonated (no charge) and the carboxylic acid group will have been deprotonated (negative charge).
- At an intermediate pH, which depends on the amino acid, the main species present is the zwitterion.
- This intermediate pH is known as the isoelectric point.
1Physical Chemistry
1.1Atomic Structure
1.1.1Fundamental Particles
1.1.2Isotopes & Mass Number
1.1.3Mass Spectrometry
1.1.4Electron Shells, Sub-Shells & Orbitals
1.1.5Electron Configuration
1.1.6Ionisation Energy
1.1.7Factors Affecting Ionisation Energies
1.1.8Trends of Ionisation
1.1.9Specific Impacts on Ionisation Energies
1.1.10End of Topic Test - Atomic Structure
1.1.11A-A* (AO3/4) - Atomic Structure
1.2Amount of Substance
1.2.1Relative Masses
1.2.2The Mole
1.2.3The Ideal Gas Equation
1.2.4Empirical & Molecular Formulae
1.2.5Balanced Equations
1.2.6Percentage Yield
1.2.7A-A* (AO3/4) - Percentage Yield
1.2.8Atom Economy
1.2.9End of Topic Test - Amount of Substance
1.2.10A-A* (AO3/4) - Substances & Yield
1.2.11Diagnostic Misconceptions - Moles
1.3Bonding
1.3.1Ionic Bonding
1.3.2Covalent & Dative Bonding
1.3.3Carbon Structures
1.3.4Metallic Bonding
1.3.5Physical Properties
1.3.6Shapes of Molecules
1.3.7Polarity
1.3.8Intermolecular Forces
1.3.9Intermolecular Forces 2
1.3.10End of Topic Test - Bonding
1.3.11Exam-Style Question - Shape of Molecules
1.3.12A-A* (AO3/4) - Bonding
1.3.13Diagnostic Misconceptions - Ions
1.3.14Diagnostic Misconceptions - Ionic & Covalent
1.3.15Diagnostic Misconceptions - Phase Change
1.3.16Diagnostic Misconceptions - Boiling
1.3.17Diagnostic Misconceptions - Polar Bonds
1.4Energetics
1.5Kinetics
1.6Equilibria
2Physical Chemistry 2 (A2 Only)
2.1Thermodynamics (A2 Only)
2.2Rate Equations (A2 Only)
2.3The Equilibrium Constant Kp (A2 Only)
2.4Electrochemical Cells (A2 Only)
2.5Acids & Bases (A2 Only)
2.5.1Brønsted-Lowry Acids & Bases (A2 Only)
2.5.2pH (A2 Only)
2.5.3The Ionic Product of Water (A2 Only)
2.5.4Weak Acids & Bases (A2 Only)
2.5.5pH Curves & Titrations (A2 Only)
2.5.6pH Curves & Titrations 2 (A2 Only)
2.5.7Buffer Solutions (A2 Only)
2.5.8End of Topic Test - Acids & Bases
2.5.9Exam-Style Question - Weak Acids
2.5.10A-A* (AO3/4) - Acids & Bases
2.5.11Diagnostic Misconceptions - Ammonia is an Alkali
2.5.12Diagnostic Misconceptions - Water's Neutrality
2.5.13Diagnostic Misconceptions - Concentrate & Strength
3Inorganic Chemistry
3.1Periodicity & Trends
4Inorganic Chemistry 2 (A2 Only)
4.1Period 3 (A2 Only)
4.2Transition Metals (A2 Only)
4.2.1General Properties (A2 Only)
4.2.2Substitution Reactions (A2 Only)
4.2.3Shapes of Complex Ions (A2 Only)
4.2.4Colours of Ions (A2 Only)
4.2.5Variable Oxidation States (A2 Only)
4.2.6Titrations (A2 Only)
4.2.7Homogeneous Catalysts (A2 Only)
4.2.8Heterogeneous Catalysts (A2 Only)
4.2.9End of Topic Test - Transition Metals
4.2.10A-A* (AO3/4) - Transition Metals
4.3Reactions of Ions in Aqueous Solutions (A2 Only)
5Organic Chemistry 1
5.1Introduction
5.2Alkanes
5.3Halogenoalkanes
5.4Alkenes
5.5Alcohols
5.6Organic Analysis
5.7A-A* (AO3/4) - Organic 1
6Organic Chemistry 2 (A2 Only)
6.1Optical Isomerism (A2 Only)
6.2Aldehydes & Ketones (A2 Only)
6.3Carboxylic Acids & Esters (A2 Only)
6.4Aromatic Chemistry (A2 Only)
6.5Amines (A2 Only)
6.6Polymers (A2 Only)
6.7Biological Organic (A2 Only)
6.8Organic Synthesis (A2 Only)
6.9NMR Spectroscopy (A2 Only)
6.10Chromatography (A2 Only)
6.11A-A* (AO3/4) - Organic 2
Jump to other topics
1Physical Chemistry
1.1Atomic Structure
1.1.1Fundamental Particles
1.1.2Isotopes & Mass Number
1.1.3Mass Spectrometry
1.1.4Electron Shells, Sub-Shells & Orbitals
1.1.5Electron Configuration
1.1.6Ionisation Energy
1.1.7Factors Affecting Ionisation Energies
1.1.8Trends of Ionisation
1.1.9Specific Impacts on Ionisation Energies
1.1.10End of Topic Test - Atomic Structure
1.1.11A-A* (AO3/4) - Atomic Structure
1.2Amount of Substance
1.2.1Relative Masses
1.2.2The Mole
1.2.3The Ideal Gas Equation
1.2.4Empirical & Molecular Formulae
1.2.5Balanced Equations
1.2.6Percentage Yield
1.2.7A-A* (AO3/4) - Percentage Yield
1.2.8Atom Economy
1.2.9End of Topic Test - Amount of Substance
1.2.10A-A* (AO3/4) - Substances & Yield
1.2.11Diagnostic Misconceptions - Moles
1.3Bonding
1.3.1Ionic Bonding
1.3.2Covalent & Dative Bonding
1.3.3Carbon Structures
1.3.4Metallic Bonding
1.3.5Physical Properties
1.3.6Shapes of Molecules
1.3.7Polarity
1.3.8Intermolecular Forces
1.3.9Intermolecular Forces 2
1.3.10End of Topic Test - Bonding
1.3.11Exam-Style Question - Shape of Molecules
1.3.12A-A* (AO3/4) - Bonding
1.3.13Diagnostic Misconceptions - Ions
1.3.14Diagnostic Misconceptions - Ionic & Covalent
1.3.15Diagnostic Misconceptions - Phase Change
1.3.16Diagnostic Misconceptions - Boiling
1.3.17Diagnostic Misconceptions - Polar Bonds
1.4Energetics
1.5Kinetics
1.6Equilibria
2Physical Chemistry 2 (A2 Only)
2.1Thermodynamics (A2 Only)
2.2Rate Equations (A2 Only)
2.3The Equilibrium Constant Kp (A2 Only)
2.4Electrochemical Cells (A2 Only)
2.5Acids & Bases (A2 Only)
2.5.1Brønsted-Lowry Acids & Bases (A2 Only)
2.5.2pH (A2 Only)
2.5.3The Ionic Product of Water (A2 Only)
2.5.4Weak Acids & Bases (A2 Only)
2.5.5pH Curves & Titrations (A2 Only)
2.5.6pH Curves & Titrations 2 (A2 Only)
2.5.7Buffer Solutions (A2 Only)
2.5.8End of Topic Test - Acids & Bases
2.5.9Exam-Style Question - Weak Acids
2.5.10A-A* (AO3/4) - Acids & Bases
2.5.11Diagnostic Misconceptions - Ammonia is an Alkali
2.5.12Diagnostic Misconceptions - Water's Neutrality
2.5.13Diagnostic Misconceptions - Concentrate & Strength
3Inorganic Chemistry
3.1Periodicity & Trends
4Inorganic Chemistry 2 (A2 Only)
4.1Period 3 (A2 Only)
4.2Transition Metals (A2 Only)
4.2.1General Properties (A2 Only)
4.2.2Substitution Reactions (A2 Only)
4.2.3Shapes of Complex Ions (A2 Only)
4.2.4Colours of Ions (A2 Only)
4.2.5Variable Oxidation States (A2 Only)
4.2.6Titrations (A2 Only)
4.2.7Homogeneous Catalysts (A2 Only)
4.2.8Heterogeneous Catalysts (A2 Only)
4.2.9End of Topic Test - Transition Metals
4.2.10A-A* (AO3/4) - Transition Metals
4.3Reactions of Ions in Aqueous Solutions (A2 Only)
5Organic Chemistry 1
5.1Introduction
5.2Alkanes
5.3Halogenoalkanes
5.4Alkenes
5.5Alcohols
5.6Organic Analysis
5.7A-A* (AO3/4) - Organic 1
6Organic Chemistry 2 (A2 Only)
6.1Optical Isomerism (A2 Only)
6.2Aldehydes & Ketones (A2 Only)
6.3Carboxylic Acids & Esters (A2 Only)
6.4Aromatic Chemistry (A2 Only)
6.5Amines (A2 Only)
6.6Polymers (A2 Only)
6.7Biological Organic (A2 Only)
6.8Organic Synthesis (A2 Only)
6.9NMR Spectroscopy (A2 Only)
6.10Chromatography (A2 Only)
6.11A-A* (AO3/4) - Organic 2
Unlock your full potential with Seneca Premium
Unlimited access to 10,000+ open-ended exam questions
Mini-mock exams based on your study history
Unlock 800+ premium courses & e-books