5.4.2
Reactions of Alkenes
Alkenes
Alkenes
Alkenes are molecules with a double covalent bond. There is a high electron density between the carbon atoms because of the double bond.


Reactions of alkenes
Reactions of alkenes
- Alkenes react with several reagents in addition reactions.
- The alkenes are nucleophiles because of the high electron density between the carbon atoms.
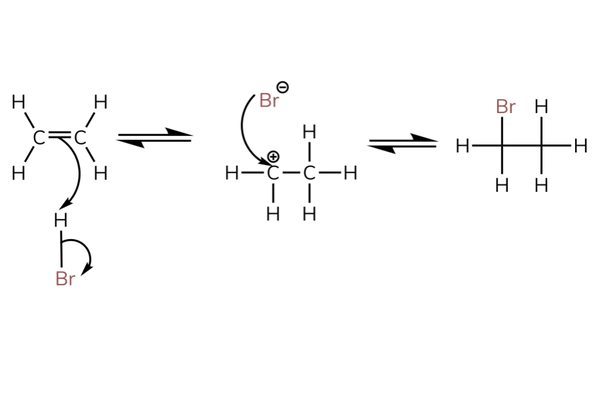
- The mechanism is shown next for the reaction with hydrogen bromide.
- The mechanism is called the electrophilic addition mechanism (because an electrophile is added).
- They will react in a similar way with sulfuric acid, and bromine.

Electrophilic addition
Electrophilic addition
- Above shows the reaction with HBr.
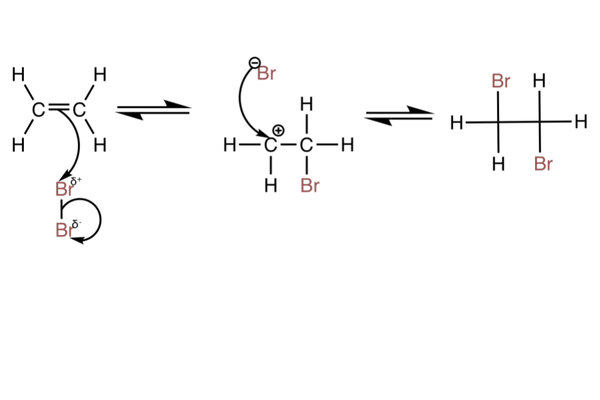

With bromine
With bromine
- The reaction can also be done with Br2.
- The partial charges are assigned to the bromine atoms because the high electron density of the double bond repels the bonding electrons and polarises the bond - this is shown in the mechanism.
Uses of Electrophilic Addition
Uses of Electrophilic Addition
Electrophilic addition reactions between alkenes and bromine as well as alkenes and sulfuric acid are useful.


With bromine
With bromine
- The reaction with bromine is used as a test for alkenes.
- Bromine water is a light orange colour and will be decolourised in the presence of an alkene (light orange → colourless).
- This test works because bromine can add to alkenes via an electrophilic addition reaction.


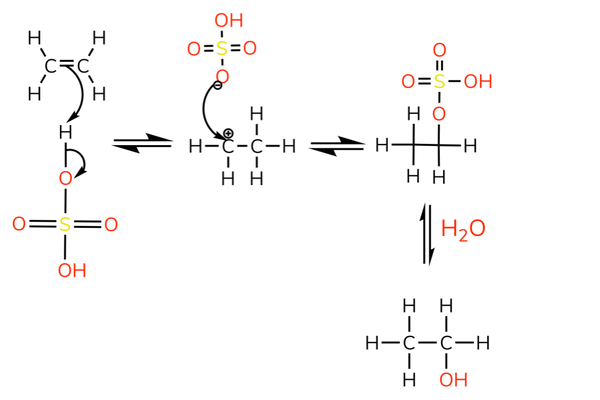
With sulfuric acid
With sulfuric acid
- The reaction with sulfuric acid is used to produce alcohols.
- The sulfate ion is removed by nucleophilic substitution.
Asymmetric Electrophilic Addition
Asymmetric Electrophilic Addition
Asymmetric alkenes can have multiple products in electrophilic addition reactions.
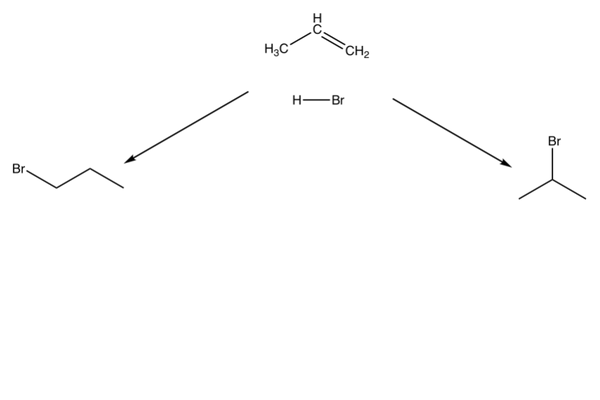

Multiple products
Multiple products
- If you have an asymmetric alkene, you can get multiple products.
- A reaction will favour one of the products over the other - we call this selectivity.
- This is shown for the reaction of propene with HBr.
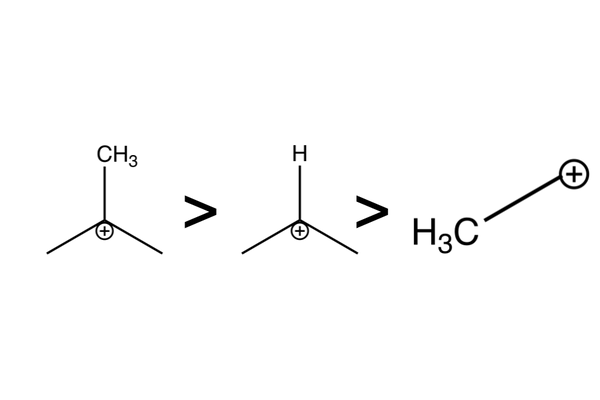

Intermediate stability
Intermediate stability
- This selectivity is driven by carbocation intermediate stability:
- Tertiary carbocations > secondary carbocations > primary carbocations.
- The more alkyl groups there are next to the positive charge, the more stable the intermediate is.
- Alkyl groups push electrons and so help to stabilise adjacent positive charge.


Which product when?
Which product when?
- When considering which product is most likely, we must consider the intermediates formed.
- 2-bromopropane is the most likely product because the intermediate is a secondary carbocation, rather than a primary carbocation.
- This is because the secondary carbocation is more stable.
- It has more alkyl groups pushing electrons onto the positive charge.
- This is because the secondary carbocation is more stable.
- Both reactions will happen and you will get both products, but you will have one major and one minor.
1Physical Chemistry
1.1Atomic Structure
1.1.1Fundamental Particles
1.1.2Isotopes & Mass Number
1.1.3Mass Spectrometry
1.1.4Electron Shells, Sub-Shells & Orbitals
1.1.5Electron Configuration
1.1.6Ionisation Energy
1.1.7Factors Affecting Ionisation Energies
1.1.8Trends of Ionisation
1.1.9Specific Impacts on Ionisation Energies
1.1.10End of Topic Test - Atomic Structure
1.1.11A-A* (AO3/4) - Atomic Structure
1.2Amount of Substance
1.2.1Relative Masses
1.2.2The Mole
1.2.3The Ideal Gas Equation
1.2.4Empirical & Molecular Formulae
1.2.5Balanced Equations
1.2.6Percentage Yield
1.2.7A-A* (AO3/4) - Percentage Yield
1.2.8Atom Economy
1.2.9End of Topic Test - Amount of Substance
1.2.10A-A* (AO3/4) - Substances & Yield
1.2.11Diagnostic Misconceptions - Moles
1.3Bonding
1.3.1Ionic Bonding
1.3.2Covalent & Dative Bonding
1.3.3Carbon Structures
1.3.4Metallic Bonding
1.3.5Physical Properties
1.3.6Shapes of Molecules
1.3.7Polarity
1.3.8Intermolecular Forces
1.3.9Intermolecular Forces 2
1.3.10End of Topic Test - Bonding
1.3.11Exam-Style Question - Shape of Molecules
1.3.12A-A* (AO3/4) - Bonding
1.3.13Diagnostic Misconceptions - Ions
1.3.14Diagnostic Misconceptions - Ionic & Covalent
1.3.15Diagnostic Misconceptions - Phase Change
1.3.16Diagnostic Misconceptions - Boiling
1.3.17Diagnostic Misconceptions - Polar Bonds
1.4Energetics
1.5Kinetics
1.6Equilibria
2Physical Chemistry 2 (A2 Only)
2.1Thermodynamics (A2 Only)
2.2Rate Equations (A2 Only)
2.3The Equilibrium Constant Kp (A2 Only)
2.4Electrochemical Cells (A2 Only)
2.5Acids & Bases (A2 Only)
2.5.1Brønsted-Lowry Acids & Bases (A2 Only)
2.5.2pH (A2 Only)
2.5.3The Ionic Product of Water (A2 Only)
2.5.4Weak Acids & Bases (A2 Only)
2.5.5pH Curves & Titrations (A2 Only)
2.5.6pH Curves & Titrations 2 (A2 Only)
2.5.7Buffer Solutions (A2 Only)
2.5.8End of Topic Test - Acids & Bases
2.5.9Exam-Style Question - Weak Acids
2.5.10A-A* (AO3/4) - Acids & Bases
2.5.11Diagnostic Misconceptions - Ammonia is an Alkali
2.5.12Diagnostic Misconceptions - Water's Neutrality
2.5.13Diagnostic Misconceptions - Concentrate & Strength
3Inorganic Chemistry
3.1Periodicity & Trends
4Inorganic Chemistry 2 (A2 Only)
4.1Period 3 (A2 Only)
4.2Transition Metals (A2 Only)
4.2.1General Properties (A2 Only)
4.2.2Substitution Reactions (A2 Only)
4.2.3Shapes of Complex Ions (A2 Only)
4.2.4Colours of Ions (A2 Only)
4.2.5Variable Oxidation States (A2 Only)
4.2.6Titrations (A2 Only)
4.2.7Homogeneous Catalysts (A2 Only)
4.2.8Heterogeneous Catalysts (A2 Only)
4.2.9End of Topic Test - Transition Metals
4.2.10A-A* (AO3/4) - Transition Metals
4.3Reactions of Ions in Aqueous Solutions (A2 Only)
5Organic Chemistry 1
5.1Introduction
5.2Alkanes
5.3Halogenoalkanes
5.4Alkenes
5.5Alcohols
5.6Organic Analysis
5.7A-A* (AO3/4) - Organic 1
6Organic Chemistry 2 (A2 Only)
6.1Optical Isomerism (A2 Only)
6.2Aldehydes & Ketones (A2 Only)
6.3Carboxylic Acids & Esters (A2 Only)
6.4Aromatic Chemistry (A2 Only)
6.5Amines (A2 Only)
6.6Polymers (A2 Only)
6.7Biological Organic (A2 Only)
6.8Organic Synthesis (A2 Only)
6.9NMR Spectroscopy (A2 Only)
6.10Chromatography (A2 Only)
6.11A-A* (AO3/4) - Organic 2
Jump to other topics
1Physical Chemistry
1.1Atomic Structure
1.1.1Fundamental Particles
1.1.2Isotopes & Mass Number
1.1.3Mass Spectrometry
1.1.4Electron Shells, Sub-Shells & Orbitals
1.1.5Electron Configuration
1.1.6Ionisation Energy
1.1.7Factors Affecting Ionisation Energies
1.1.8Trends of Ionisation
1.1.9Specific Impacts on Ionisation Energies
1.1.10End of Topic Test - Atomic Structure
1.1.11A-A* (AO3/4) - Atomic Structure
1.2Amount of Substance
1.2.1Relative Masses
1.2.2The Mole
1.2.3The Ideal Gas Equation
1.2.4Empirical & Molecular Formulae
1.2.5Balanced Equations
1.2.6Percentage Yield
1.2.7A-A* (AO3/4) - Percentage Yield
1.2.8Atom Economy
1.2.9End of Topic Test - Amount of Substance
1.2.10A-A* (AO3/4) - Substances & Yield
1.2.11Diagnostic Misconceptions - Moles
1.3Bonding
1.3.1Ionic Bonding
1.3.2Covalent & Dative Bonding
1.3.3Carbon Structures
1.3.4Metallic Bonding
1.3.5Physical Properties
1.3.6Shapes of Molecules
1.3.7Polarity
1.3.8Intermolecular Forces
1.3.9Intermolecular Forces 2
1.3.10End of Topic Test - Bonding
1.3.11Exam-Style Question - Shape of Molecules
1.3.12A-A* (AO3/4) - Bonding
1.3.13Diagnostic Misconceptions - Ions
1.3.14Diagnostic Misconceptions - Ionic & Covalent
1.3.15Diagnostic Misconceptions - Phase Change
1.3.16Diagnostic Misconceptions - Boiling
1.3.17Diagnostic Misconceptions - Polar Bonds
1.4Energetics
1.5Kinetics
1.6Equilibria
2Physical Chemistry 2 (A2 Only)
2.1Thermodynamics (A2 Only)
2.2Rate Equations (A2 Only)
2.3The Equilibrium Constant Kp (A2 Only)
2.4Electrochemical Cells (A2 Only)
2.5Acids & Bases (A2 Only)
2.5.1Brønsted-Lowry Acids & Bases (A2 Only)
2.5.2pH (A2 Only)
2.5.3The Ionic Product of Water (A2 Only)
2.5.4Weak Acids & Bases (A2 Only)
2.5.5pH Curves & Titrations (A2 Only)
2.5.6pH Curves & Titrations 2 (A2 Only)
2.5.7Buffer Solutions (A2 Only)
2.5.8End of Topic Test - Acids & Bases
2.5.9Exam-Style Question - Weak Acids
2.5.10A-A* (AO3/4) - Acids & Bases
2.5.11Diagnostic Misconceptions - Ammonia is an Alkali
2.5.12Diagnostic Misconceptions - Water's Neutrality
2.5.13Diagnostic Misconceptions - Concentrate & Strength
3Inorganic Chemistry
3.1Periodicity & Trends
4Inorganic Chemistry 2 (A2 Only)
4.1Period 3 (A2 Only)
4.2Transition Metals (A2 Only)
4.2.1General Properties (A2 Only)
4.2.2Substitution Reactions (A2 Only)
4.2.3Shapes of Complex Ions (A2 Only)
4.2.4Colours of Ions (A2 Only)
4.2.5Variable Oxidation States (A2 Only)
4.2.6Titrations (A2 Only)
4.2.7Homogeneous Catalysts (A2 Only)
4.2.8Heterogeneous Catalysts (A2 Only)
4.2.9End of Topic Test - Transition Metals
4.2.10A-A* (AO3/4) - Transition Metals
4.3Reactions of Ions in Aqueous Solutions (A2 Only)
5Organic Chemistry 1
5.1Introduction
5.2Alkanes
5.3Halogenoalkanes
5.4Alkenes
5.5Alcohols
5.6Organic Analysis
5.7A-A* (AO3/4) - Organic 1
6Organic Chemistry 2 (A2 Only)
6.1Optical Isomerism (A2 Only)
6.2Aldehydes & Ketones (A2 Only)
6.3Carboxylic Acids & Esters (A2 Only)
6.4Aromatic Chemistry (A2 Only)
6.5Amines (A2 Only)
6.6Polymers (A2 Only)
6.7Biological Organic (A2 Only)
6.8Organic Synthesis (A2 Only)
6.9NMR Spectroscopy (A2 Only)
6.10Chromatography (A2 Only)
6.11A-A* (AO3/4) - Organic 2
Unlock your full potential with Seneca Premium
Unlimited access to 10,000+ open-ended exam questions
Mini-mock exams based on your study history
Unlock 800+ premium courses & e-books