3.3.2
Enzymes
Enzymes
Enzymes
Enzymes are proteins that catalyse reactions. The chemical reactants that enzymes bind to are called substrates.


Biological catalyst
Biological catalyst
- A substance that speeds up a chemical reaction without being used up itself is a catalyst.
- Enzymes are proteins that catalyse biochemical reactions.
- Enzymes can act inside or outside of cells.
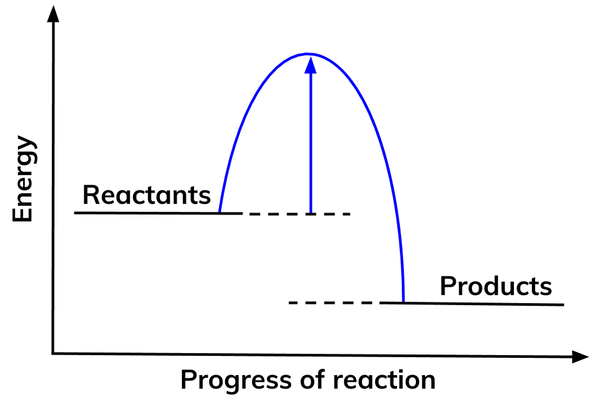

Activation energy
Activation energy
- For a chemical reaction to start, it needs a specific amount of energy. This amount of energy is different for different reactions.
- This is called the activation energy.
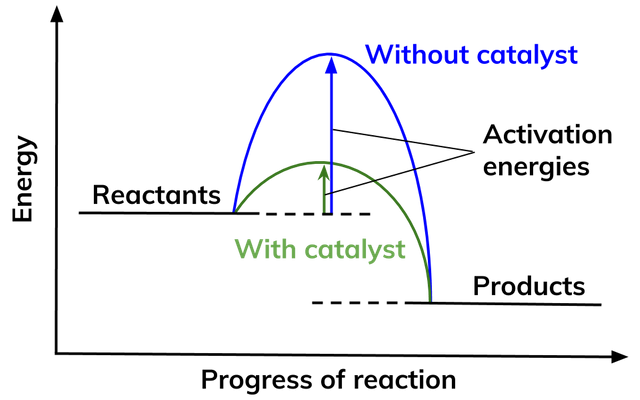

Activation energy and enzymes
Activation energy and enzymes
- Enzymes lower the activation energies of chemical reactions inside the cell to increase the rate of reactions.
- Enzymes lower the activation energy by binding to the reactant molecules (substrate) and allowing chemical bond-breaking and bond-forming processes to happen more easily.
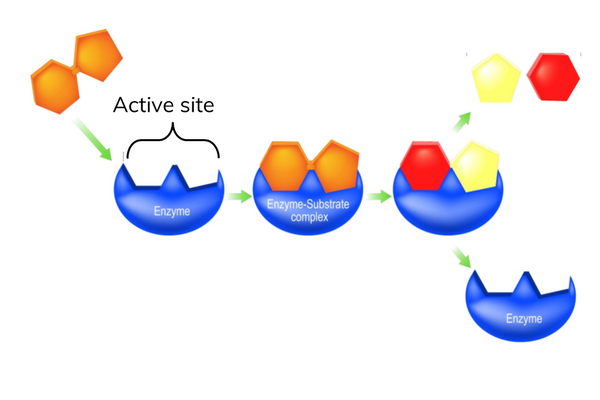

The active site
The active site
- Enzymes catalyse specific reactions.
- The active site has a specific shape for each enzyme.
- Substrates with a complementary shape to the active site of an enzyme can bind to form an enzyme-substrate complex.
- The shape of the active site is determined by the tertiary structure of the polypeptide.
Models of Enzyme Action
Models of Enzyme Action
Models of enzyme action have changed over time. For many years, it was thought that enzymes worked in a lock and key manner. The induced fit model is now more widely accepted.
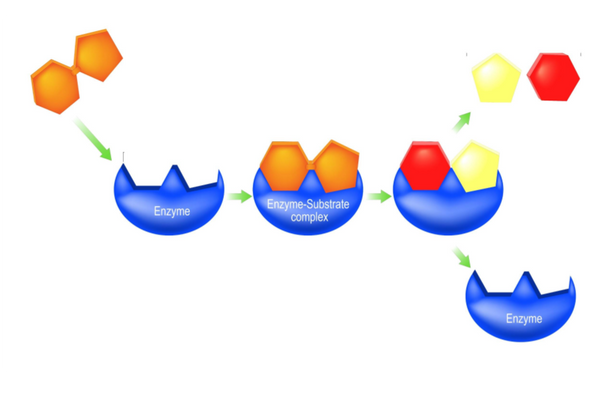

The lock and key model
The lock and key model
- The lock and key model was originally used to explain enzyme action.
- The model proposes that the enzyme and substrate fit together perfectly.
- The substrate is a key fitting into a lock (the enzyme).
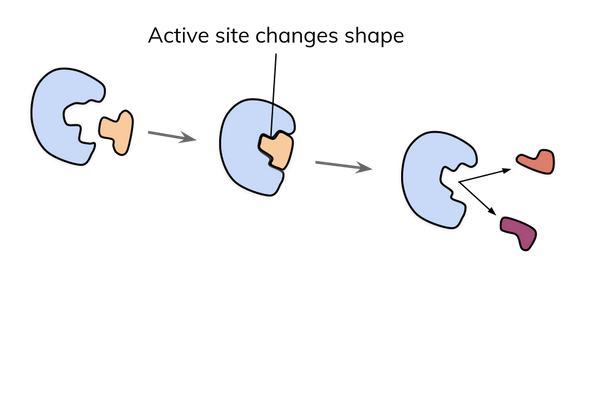

The induced fit model
The induced fit model
- The induced fit model suggests there is a more dynamic interaction between enzyme and substrate.
- The model states that as an enzyme and substrate come together, their interaction causes a small shift in the enzyme’s structure.
- The shift means that the enzyme and substrate can bind to form an enzyme-substrate complex and catalyse a reaction.
- This model is now more widely accepted.
Enzyme-Substrate Complexes
Enzyme-Substrate Complexes
Enzymes bind with substrates to form an enzyme-substrate complex in a specific fashion. The specificity of enzymes is determined by two things:


Active site
Active site
- Every enzyme only catalyses one specific reaction.
- Every enzyme has a specific active site that is complementary to the specific substrate.
- This jigsaw puzzle-like match between an enzyme and its substrates is what makes enzymes highly specific.
,h_400,q_80,w_640.png)
,h_400,q_80,w_640.png)
Environmental factors
Environmental factors
- The 3D, tertiary structure of the polypetide chain determines the shape of the active site.
- Environmental changes can change the tertiary structure of the active site and can stop the enzyme from working properly.
- This is called a denatured enzyme.
1Principles of Science I
1.1Structure & Bonding
1.1.1Atomic Model
1.1.2Electron Shells, Sub-Shells & Orbitals
1.1.3Ionic Bonding
1.1.4Representing Ionic Bonds
1.1.5Covalent Bonding
1.1.6Representing Covalent Bonds
1.1.7Metallic Bonding
1.1.8Intermolecular Forces
1.1.9Intermolecular Forces 2
1.1.10End of Topic Test - Bonding
1.1.11Relative Masses
1.1.12The Mole
1.1.13Molar Calculations
1.1.14Molar Calculations 2
1.1.15Empirical & Molecular Formulae
1.1.16Balanced Equations
1.1.17Percentage Yield
1.1.18End of Topic Test - Amount of Substance
1.2Properties of Substances
1.2.1The Periodic Table
1.2.2Ionisation Energy
1.2.3Factors Affecting Ionisation Energies
1.2.4Trends of Ionisation
1.2.5Trends in the Periodic Table
1.2.6Polarity
1.2.7Metals & Non-Metals
1.2.8Alkali Metals
1.2.9Alkaline Earth Metals
1.2.10Reactivity of Alkaline Earth Metals
1.2.11Redox
1.2.12Transition Metals
1.2.13Redox Reactions of Transition Metals
1.3Cell Structure & Function
1.4Cell Specialisation
1.5Tissue Structure & Function
1.5.1Human Gas Exchange
1.5.2Blood Vessels
1.5.3Atherosclerosis
1.5.4Skeletal Muscle
1.5.5Slow & Fast Twitch Fibres
1.5.6Neurones
1.5.7Speed of Transmission
1.5.8Action Potentials
1.5.9End of Topic Test - Neurones & Action Potentials
1.5.10Synapses
1.5.11Types of Synapse
1.5.12Medical Application
1.5.13End of Topic Test - Synapses
1.5.14Chemical Brain Imbalances
1.5.15Effect of Drugs on the Brain
1.6Working with Waves
1.7Waves in Communication
2Practical Scientific Procedures and Techniques
3Science Investigation Skills
3.1Scientific Processes
3.2Data Handling & Analysis
3.3Enzymes in Action
3.4Diffusion
3.5Plants & Their Environment
3.6Energy Content in Fuels
4Principles of Science II
4.1Extracting Elements
4.2Relating Properties to use of Substances
4.3Organic Chemistry
4.4Energy Changes in Industry
4.5The Circulatory System
4.5.1The Circulatory System
4.5.2Blood Vessels
4.5.3Blood Transfusion & the ABO Rhesus System
4.5.4The Heart
4.5.5The Cardiac Cycle
4.5.6Cardiac Output
4.5.7Coordination of Heart Action
4.5.8Heart Dissection
4.5.9Controlling Heart Rate
4.5.10Electrocardiograms
4.5.11Cardiovascular Disease
4.5.12Investigating Heart Rates
4.6Ventilation & Gas Exchange
4.7Urinary System
4.9Thermal Physics
4.9.1Power & Efficiency
4.9.2Work & Energy
4.9.3Conservation of Energy
4.9.4Pressure
4.9.5First Law of Thermodynamics
4.9.6Second Law of Thermodynamics
4.9.7Heat Engines, Heat Pumps & Refrigerators
4.9.8Non-Flow Processes
4.9.9p-V Diagrams
4.9.10Ideal Gases
4.9.11Ideal Gases 2
4.9.12Thermal Energy Transfer
4.9.13Thermal Energy Transfer Experiments
4.10Materials
5Contemporary Issues in Science
5.1Contemporary Issues in Science
5.2Analysing Scientific Information
Jump to other topics
1Principles of Science I
1.1Structure & Bonding
1.1.1Atomic Model
1.1.2Electron Shells, Sub-Shells & Orbitals
1.1.3Ionic Bonding
1.1.4Representing Ionic Bonds
1.1.5Covalent Bonding
1.1.6Representing Covalent Bonds
1.1.7Metallic Bonding
1.1.8Intermolecular Forces
1.1.9Intermolecular Forces 2
1.1.10End of Topic Test - Bonding
1.1.11Relative Masses
1.1.12The Mole
1.1.13Molar Calculations
1.1.14Molar Calculations 2
1.1.15Empirical & Molecular Formulae
1.1.16Balanced Equations
1.1.17Percentage Yield
1.1.18End of Topic Test - Amount of Substance
1.2Properties of Substances
1.2.1The Periodic Table
1.2.2Ionisation Energy
1.2.3Factors Affecting Ionisation Energies
1.2.4Trends of Ionisation
1.2.5Trends in the Periodic Table
1.2.6Polarity
1.2.7Metals & Non-Metals
1.2.8Alkali Metals
1.2.9Alkaline Earth Metals
1.2.10Reactivity of Alkaline Earth Metals
1.2.11Redox
1.2.12Transition Metals
1.2.13Redox Reactions of Transition Metals
1.3Cell Structure & Function
1.4Cell Specialisation
1.5Tissue Structure & Function
1.5.1Human Gas Exchange
1.5.2Blood Vessels
1.5.3Atherosclerosis
1.5.4Skeletal Muscle
1.5.5Slow & Fast Twitch Fibres
1.5.6Neurones
1.5.7Speed of Transmission
1.5.8Action Potentials
1.5.9End of Topic Test - Neurones & Action Potentials
1.5.10Synapses
1.5.11Types of Synapse
1.5.12Medical Application
1.5.13End of Topic Test - Synapses
1.5.14Chemical Brain Imbalances
1.5.15Effect of Drugs on the Brain
1.6Working with Waves
1.7Waves in Communication
2Practical Scientific Procedures and Techniques
3Science Investigation Skills
3.1Scientific Processes
3.2Data Handling & Analysis
3.3Enzymes in Action
3.4Diffusion
3.5Plants & Their Environment
3.6Energy Content in Fuels
4Principles of Science II
4.1Extracting Elements
4.2Relating Properties to use of Substances
4.3Organic Chemistry
4.4Energy Changes in Industry
4.5The Circulatory System
4.5.1The Circulatory System
4.5.2Blood Vessels
4.5.3Blood Transfusion & the ABO Rhesus System
4.5.4The Heart
4.5.5The Cardiac Cycle
4.5.6Cardiac Output
4.5.7Coordination of Heart Action
4.5.8Heart Dissection
4.5.9Controlling Heart Rate
4.5.10Electrocardiograms
4.5.11Cardiovascular Disease
4.5.12Investigating Heart Rates
4.6Ventilation & Gas Exchange
4.7Urinary System
4.9Thermal Physics
4.9.1Power & Efficiency
4.9.2Work & Energy
4.9.3Conservation of Energy
4.9.4Pressure
4.9.5First Law of Thermodynamics
4.9.6Second Law of Thermodynamics
4.9.7Heat Engines, Heat Pumps & Refrigerators
4.9.8Non-Flow Processes
4.9.9p-V Diagrams
4.9.10Ideal Gases
4.9.11Ideal Gases 2
4.9.12Thermal Energy Transfer
4.9.13Thermal Energy Transfer Experiments
4.10Materials
5Contemporary Issues in Science
5.1Contemporary Issues in Science
5.2Analysing Scientific Information
Unlock your full potential with Seneca Premium
Unlimited access to 10,000+ open-ended exam questions
Mini-mock exams based on your study history
Unlock 800+ premium courses & e-books