2.5.5
Reactions of Alkenes
Alkenes
Alkenes
Alkenes are molecules with a double covalent bond. There is a high electron density between the carbon atoms because of the double bond.


Reactions of alkenes
Reactions of alkenes
- Alkenes react with several reagents in addition reactions.
- The alkenes are nucleophiles because of the high electron density between the carbon atoms.
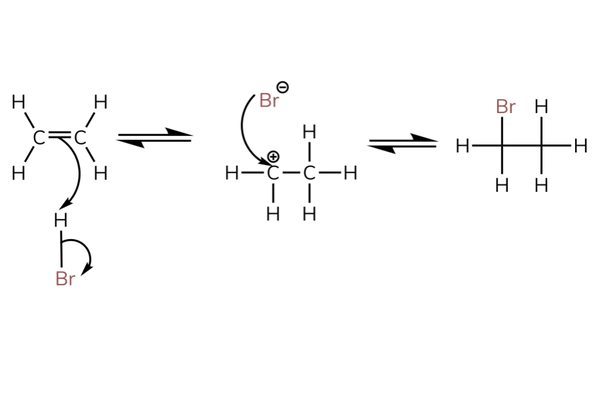
- The mechanism is shown next for the reaction with hydrogen bromide.
- The mechanism is called the electrophilic addition mechanism (because an electrophile is added).
- They will react in a similar way with sulfuric acid, and bromine.

Electrophilic addition
Electrophilic addition
- Above shows the reaction with HBr.
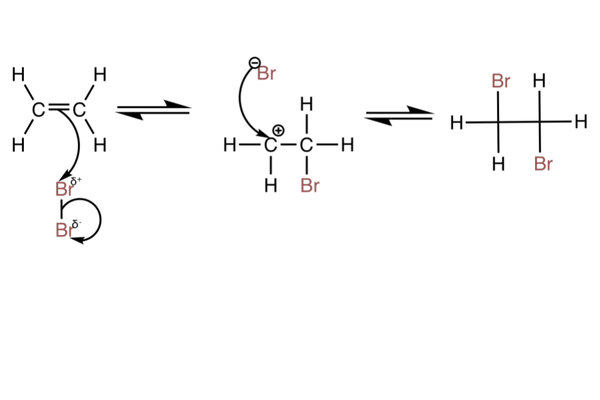

With bromine
With bromine
- The reaction can also be done with Br2.
- The partial charges are assigned to the bromine atoms because the high electron density of the double bond repels the bonding electrons and polarises the bond - this is shown in the mechanism.
Uses of Electrophilic Addition
Uses of Electrophilic Addition
Electrophilic addition reactions between alkenes and bromine as well as alkenes and sulfuric acid are useful.


With bromine
With bromine
- The reaction with bromine is used as a test for alkenes.
- Bromine water is a light orange colour and will be decolourised in the presence of an alkene (light orange → colourless).
- This test works because bromine can add to alkenes via an electrophilic addition reaction.


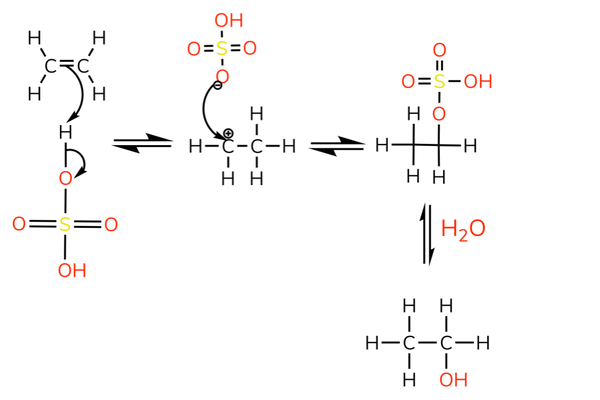
With sulfuric acid
With sulfuric acid
- The reaction with sulfuric acid is used to produce alcohols.
- The sulfate ion is removed by nucleophilic substitution.
Asymmetric Electrophilic Addition
Asymmetric Electrophilic Addition
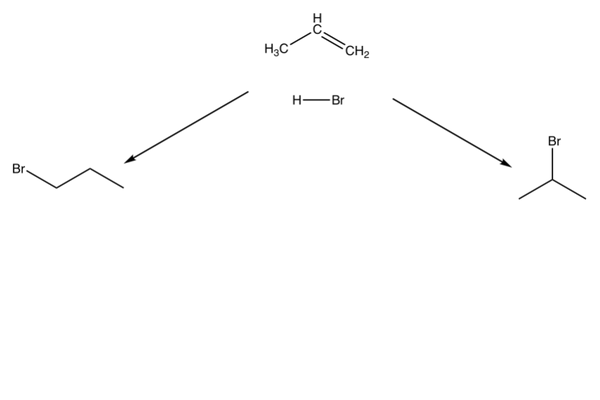
Asymmetric alkenes can have multiple products in electrophilic addition reactions.

Multiple products
Multiple products
- If you have an asymmetric alkene, you can get multiple products.
- A reaction will favour one of the products over the other - we call this selectivity.
- This is shown for the reaction of propene with HBr.
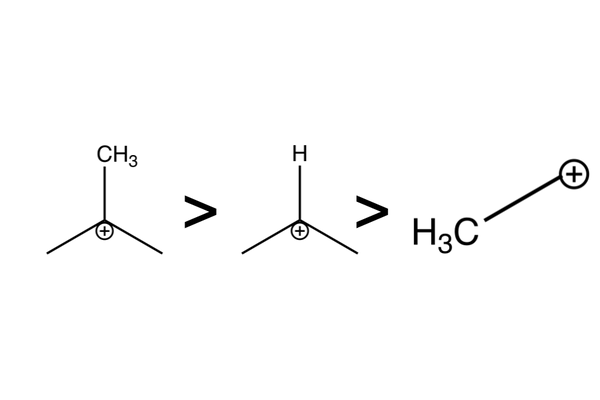

Intermediate stability
Intermediate stability
- This selectivity is driven by carbocation intermediate stability:
- Tertiary carbocations > secondary carbocations > primary carbocations.
- The more alkyl groups there are next to the positive charge, the more stable the intermediate is.
- Alkyl groups push electrons and so help to stabilise adjacent positive charge.


Which product when?
Which product when?
- When considering which product is most likely, we must consider the intermediates formed.
- 2-bromopropane is the most likely product because the intermediate is a secondary carbocation, rather than a primary carbocation.
- This is because the secondary carbocation is more stable.
- It has more alkyl groups pushing electrons onto the positive charge.
- This is because the secondary carbocation is more stable.
- Both reactions will happen and you will get both products, but you will have one major and one minor.
1Structure - Models of the Particulate of Matter
1.1Introduction to the Particulate Model of Matter
1.2The Nuclear Atom
1.3Electron Configuration
1.4Counting Particles by Mass: The Mole
1.6Elements, Compounds & Mixtures
1.7States of Matter & Changes of State
1.8Reacting Masses &. Volumes
1.9Solutions
2Structure - Models of Bonding & Structure
2.1The Ionic Model
2.2The Covalent Model
2.3Covalent Structures
2.4The Metallic Model
2.5From Models to Materials
2.6Valence Electrons & Ionic Compounds
2.7Molecular Shape
3Structure - Classification of Matter
3.1The Periodic Table: Classification of Elements
3.2Periodic Trends
3.3Group 1 Alkali Metals
3.4Halogens
3.5Noble gases, group 18
3.6Functional Groups: Classification of Organic
3.7Functional Group Chemistry
3.8Alkanes
3.9Alcohols
4Reactivity - What Drives Chemical Reaction?
4.1Endothermic & Exothermic Reactions
4.2Enthalpy of Reaction, Formation, & Hess' Law
5Reactivity - How Much, How Fast & How Far?
5.1Kinetics
5.2Rates of Reaction
5.3Stoichometry
5.4Le Châtelier’s Principle
5.5Introduction to Equilibrium
5.6Equilibrium Constant
5.7Reaction Quotient & Equilibrium Constant
6Reactivity - The Mechanisms of Chemical Change
6.1Proton Transfer Reactions
6.2The pH Scale
6.3Strong & Weak Acids and Bases
6.4Acid Deposition
6.5Types of Organic Reactions
6.6Oxidation & Reduction
6.7Electrochemical Cells
6.9Acid-Base Titrations
6.9.1Titration Calculation Weak Acid & Strong Base
6.9.2Titration Experimental Detail
6.9.3Extended Response - Titration
6.9.4Titration Calculations
6.9.5Titration Curves
6.9.6Titration Calculation Strong Acid & Weak Base
6.9.7IB Multiple Choice - Titrations
6.9.8Polyprotic Acids
6.9.9Titration Calculations Strong Acid & Strong Base
6.9.10Titrations Curves 2
7Measurement, Data Processing & Analysis
7.1Uncertainties & Errors in Measurements & Results
7.2Graphical Techniques
7.3Spectroscopic Identification of Organic Compounds
7.4Infrared Spectroscpy
Jump to other topics
1Structure - Models of the Particulate of Matter
1.1Introduction to the Particulate Model of Matter
1.2The Nuclear Atom
1.3Electron Configuration
1.4Counting Particles by Mass: The Mole
1.6Elements, Compounds & Mixtures
1.7States of Matter & Changes of State
1.8Reacting Masses &. Volumes
1.9Solutions
2Structure - Models of Bonding & Structure
2.1The Ionic Model
2.2The Covalent Model
2.3Covalent Structures
2.4The Metallic Model
2.5From Models to Materials
2.6Valence Electrons & Ionic Compounds
2.7Molecular Shape
3Structure - Classification of Matter
3.1The Periodic Table: Classification of Elements
3.2Periodic Trends
3.3Group 1 Alkali Metals
3.4Halogens
3.5Noble gases, group 18
3.6Functional Groups: Classification of Organic
3.7Functional Group Chemistry
3.8Alkanes
3.9Alcohols
4Reactivity - What Drives Chemical Reaction?
4.1Endothermic & Exothermic Reactions
4.2Enthalpy of Reaction, Formation, & Hess' Law
5Reactivity - How Much, How Fast & How Far?
5.1Kinetics
5.2Rates of Reaction
5.3Stoichometry
5.4Le Châtelier’s Principle
5.5Introduction to Equilibrium
5.6Equilibrium Constant
5.7Reaction Quotient & Equilibrium Constant
6Reactivity - The Mechanisms of Chemical Change
6.1Proton Transfer Reactions
6.2The pH Scale
6.3Strong & Weak Acids and Bases
6.4Acid Deposition
6.5Types of Organic Reactions
6.6Oxidation & Reduction
6.7Electrochemical Cells
6.9Acid-Base Titrations
6.9.1Titration Calculation Weak Acid & Strong Base
6.9.2Titration Experimental Detail
6.9.3Extended Response - Titration
6.9.4Titration Calculations
6.9.5Titration Curves
6.9.6Titration Calculation Strong Acid & Weak Base
6.9.7IB Multiple Choice - Titrations
6.9.8Polyprotic Acids
6.9.9Titration Calculations Strong Acid & Strong Base
6.9.10Titrations Curves 2
7Measurement, Data Processing & Analysis
7.1Uncertainties & Errors in Measurements & Results
7.2Graphical Techniques
7.3Spectroscopic Identification of Organic Compounds
7.4Infrared Spectroscpy
Unlock your full potential with Seneca Premium
Unlimited access to 10,000+ open-ended exam questions
Mini-mock exams based on your study history
Unlock 800+ premium courses & e-books