2.2.6
The pH Scale
The pH scale
The pH scale
Acids and alkalis all have a pH, which is a number that tells you how strong the acid or alkali is. It starts at zero and goes up to 14.
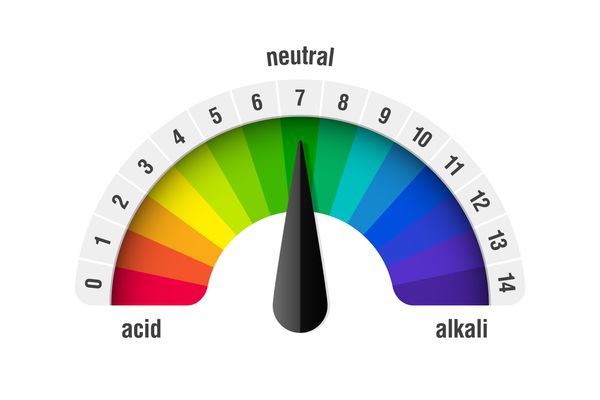

The pH scale
The pH scale
- Anything below 7 is an acid.
- The lower the number, the stronger the acid.
- Anything above 7 is an alkali.
- The higher the number, the stronger the alkali. pH 7 is neutral – these chemicals are neither acid or alkali.
- Pure water has a pH of 7.
- Each pH number is also given a colour.
- The colours on the pH scale are what colour the chemical will go if ‘universal indicator’ is added.


Common acids
Common acids
- Lemon juice.
- Hydrochloric acid.
- Sulfuric acid.
- Nitric acid.
- Vinegar.


Common alkalis
Common alkalis
- Soap.
- Toothpaste.
- Ammonia.
- Sodium hydroxide.
- Lithium hydroxide.
- Calcium oxide.
1Biology
1.1Cells, Tissues & Organs
1.1.1Microscopes
1.1.2Magnification
1.1.3Multicellular Organisms
1.1.4Tissues
1.1.5Organs
1.1.6Unicellular Organisms
1.1.7Diffusion
1.1.8Factors Affecting Diffusion
1.1.9Plant Cells
1.1.10Cellulose
1.1.11Plant Tissues
1.1.12Leaves
1.1.13Animal Cells
1.1.14Comparing Animal & Plant Cells
1.1.15How to Make a Model Animal and Plant Cell
1.1.16Specialised Cells
1.1.17Stem Cells
1.1.18Uses of Stem Cells
1.1.19Disadvantages of Stem Cells
1.1.20Blood Components
1.1.21Platelets
1.1.22End of Topic Test - Cells & Organisation
1.1.23The Lungs
1.1.24Breathing
1.1.25Plant Gas Exchange
1.1.26Drugs
1.1.27End of Topic Test - Living Organisms
1.1.28Diagnostic Misconceptions - Microbes
1.1.29Diagnostic Misconceptions - Diff. vs Specialised
1.1.30Diagnostic Misconceptions - Types of Cell
1.1.31Diagnostic Misconceptions - Plants & Animal Cells
1.2Reproduction & Variation
1.2.1Reproduction in Humans
1.2.2Male Reproductive System
1.2.3Female Reproductive System
1.2.4Gestation
1.2.5Pregnancy
1.2.6Puberty
1.2.7The Menstrual Cycle
1.2.8Reproduction in Plants
1.2.9Asexual Reproduction
1.2.10Advantages of Asexual Reproduction
1.2.11Adaptations
1.2.12Evolution
1.2.13Pollination
1.2.14Dispersal Method
1.2.15Variation
1.2.16Causes of Variation
1.2.17Inheritance
1.2.18Species & Selective Breeding
1.2.19Genetic Conditions - Cystic Fibrosis
1.2.20Genetic Conditions - Haemophilia
1.2.21End of Topic Test - Reproduction & Variation
1.2.22Diagnostic Misconceptions - Asexual
1.3Ecological Relationships & Classification
1.3.1Species Interdependence
1.3.2Food Chains & Webs
1.3.3Changes to Food Webs - Plants
1.3.4Changes to Food Webs - Animals
1.3.5Changes to Food Webs - Factors
1.3.6Relationships in an Ecosystem
1.3.7The Impact of Environmental Change
1.3.8Decomposers
1.3.9Decay
1.3.10Assessing Ecosystems
1.3.11Ecological Sampling - Quadrats
1.3.12Ecological Sampling
1.3.13Required Practical - Estimating Population Size
1.3.14Pyramids of Number and Biomass
1.3.15Classification of Living Organisms
1.3.16Competition Between Organisms
1.3.17Adaptations of Plants
1.3.18Natural Selection
1.3.19Evidence for Evolution
1.3.20Environmental Changes & Extinctions
1.3.21The Importance of Biodiversity
1.3.22Bioaccumulation
1.3.23Bioaccumulation - DDT and Mercury
1.3.24End of Topic Test - Material Cycles & Energy
1.4Digestion & Nutrition
1.4.1Balanced Diets
1.4.2Vitamins & Minerals
1.4.3Protein
1.4.4Lipids, Oils and Fats
1.4.5Carbohydrates
1.4.6Starch
1.4.7Energy Needs
1.4.8Dietary Fibre
1.4.9Diseases Caused by Nutritional Deficiencies
1.4.10Digestion
1.4.11Plant Nutrition
1.4.12Enzymes in Digestion
1.4.13Required Practical - Enzymes in Digestion
1.4.14Diagnostic Misconceptions - Digestion
1.4.15Diagnostic Misconceptions - Excretion vs Egestion
1.5Plants & Photosynthesis
1.5.1Roots
1.5.2Photosynthesis
1.5.3Leaves in Photosynthesis
1.5.4Rate of Photosynthesis
1.5.5Testing the Rate of Photosynthesis
1.5.6Water Transport in Plants
1.5.7Translocation
1.5.8The Carbon Cycle
1.5.9Human Activities & Carbon Dioxide
1.5.10Diagnostic Misconceptions - Plant Food
1.5.11Diagnostic Misconceptions - Transpiration
1.6Biological Systems & Processes
1.6.1Living Organisms
1.6.2Dichotomous Keys
1.6.3Biomechanics
1.6.4Muscles
1.6.5The Skeleton
1.6.6Bones
1.6.7Tendons and Ligaments
1.6.8Carpals and Tarsals
1.6.9Measuring Forces
1.6.10Antagonistic Muscle Pairings
1.6.11The Respiratory System
1.6.12Structure & Function of the Gas Exchange System
1.6.13Breathing
1.6.14Respiration
1.6.15Respiration During Exercise
1.6.16Anaerobic Respiration
1.6.17Lactic Acid
1.6.18Effects of Smoking on the Respiratory System
1.6.19Balanced Diets
1.6.20Human Growth & Development
1.6.21DNA
1.6.22Alleles
1.6.23Genotype vs Phenotype
1.6.24Punnett Squares
1.6.25Joints
1.6.26The Renal System
1.6.27The Double Circulatory System
1.6.28Gas Exchange
1.6.29Heart and Blood
1.6.30Blood Vessels
1.6.31Glucose
1.6.32Glucose and Diabetes
1.6.33The Effects of Recreational Drug Use
1.6.34Human Illnesses
1.6.35Bacterial and Viral Infections
1.6.36Antibiotics
1.6.37Vaccinations
1.6.38How Antibiotics and Vaccines Work
1.6.39Mental Health
1.6.40Depression and Anxiety
2Chemistry
2.1Particles
2.1.1Particles
2.1.2States of Matter
2.1.3Compression of Substances
2.1.4Shape of Substances
2.1.5Changes of State
2.1.6Elements and Atoms
2.1.7Compounds
2.1.8Diffusion
2.1.9Changing State
2.1.10Pressure
2.1.11Temperature Increase in a Gas
2.1.12Conservation of Mass
2.1.13Purity of Substances
2.1.14Pure Substances
2.1.15Evaporation
2.1.16Mixtures
2.1.17Separating Mixtures
2.1.18Distillation
2.1.19Chromatography
2.1.20Solubility
2.1.21Investigating Solubility
2.1.22Chemical Symbols
2.1.23Chemical Formulae
2.1.24Ceramics
2.1.25Polymers
2.1.26Composites
2.1.27Diagnostic Misconceptions - Particles
2.1.28Diagnostic Misconceptions - Temperature
2.1.29Diagnostic Misconceptions - Boiling Liquids
2.2Chemical Reactions
2.3Atoms, Elements, Compounds
2.4The Periodic Table
2.5Materials & the Earth
2.5.1The Composition of The Earth
2.5.2The Structure of the Earth
2.5.3Igneous Rocks
2.5.4Sedimentary Rocks
2.5.5Metamorphic Rocks
2.5.6The Rock Cycle
2.5.7Physical Weathering
2.5.8Chemical Weathering
2.5.9Biological Weathering
2.5.10The Formation of Fossils
2.5.11Crude Oil
2.5.12End of Topic Test - Earth
2.5.13The Earth's Early Atmosphere
2.5.14The Earth's Atmosphere Today
2.5.15Oxygen in the Atmosphere
2.5.16Carbon Dioxide in the Atmosphere
2.5.17Greenhouse Gases
2.5.18Climate Change
2.5.19Resources
2.5.20Recycling
2.5.21Ceramics
2.5.22Polymers
2.5.23Composites
2.5.24End of Topic Test - Materials
2.5.25End of Topic Test - Polymers
2.5.26Diagnostic Misconceptions - Air
2.5.27Diagnostic Misconceptions - Greenhouse Effect
2.6Reactivity
2.6.1Ions
2.6.2Ionic Bonding
2.6.3State Symbols
2.6.4Balancing Chemical Equations
2.6.5Relative Formula Mass
2.6.6Calculating the Relative Formula Mass
2.6.7The Reactivity Series
2.6.8Carbon & The Reactivity Series
2.6.9Displacement Reactions
2.6.10Displacement Reactions - Halogens
2.6.11Alloys
2.6.12Metal Alloys
2.7Energetics
2.8Properties of Materials
3Physics
3.1Energy
3.1.1Energy Stores & Pathways
3.1.2Energy Transfers
3.1.3Common Energy Transfers
3.1.4Wasted Energy
3.1.5Efficiency of Energy Transfer
3.1.6Sankey Diagrams
3.1.7Heat & Temperature
3.1.8Heat Transfer
3.1.9Conductors vs Insulators
3.1.10Reducing Energy Transfers
3.1.11Energy & Power
3.1.12Energy in Food
3.1.13Calories
3.1.14Food Labels
3.1.15Power Ratings of Appliances
3.1.16Comparing Bulbs
3.1.17Fuel Bills
3.1.18Calculating Fuel Bills
3.1.19Non-Renewable Energy - Fossil Fuels
3.1.20Other Non-Renewables
3.1.21Renewable Energy - Air & Ground
3.1.22Renewable Energy - Water
3.1.23Conservation of Energy
3.1.24End of Topic Test - Energy
3.1.25Diagnostic Misconceptions - Stationary Objects
3.2Forces & Motion
3.2.1Forces
3.2.2Contact Forces
3.2.3Balanced Forces
3.2.4Force Diagrams & Resultant Forces
3.2.5Free Body Diagram - Uses
3.2.6Force & Acceleration
3.2.7Gravity
3.2.8Weight
3.2.9Pressure
3.2.10Speed
3.2.11Relative Motion
3.2.12Friction 1
3.2.13Friction 2
3.2.14Turning Effect
3.2.15Moment
3.2.16Equation for Moments
3.2.17Water & Air Resistance
3.2.18Distance-Time Graphs
3.2.19Levers
3.2.20Work & Machines
3.2.21Work
3.2.22Machines
3.2.23Elasticity
3.2.24Elasticity - Hooke's Law
3.2.25Archimedes Principle
3.2.26Floating & Sinking
3.2.27End of Topic Test - Forces & Motion
3.2.28Vacuums
3.2.29Thermal Energy & Conduction
3.2.30Convection & Radiation
3.2.31Evaporation
3.2.32Diagnostic Misconceptions - Weight vs Mass
3.2.33Diagnostic Misconceptions - Gravity
3.3Waves
3.3.1Waves
3.3.2Types of Waves
3.3.3Observing Waves
3.3.4Wave Speed
3.3.5Earthquakes
3.3.6Hearing
3.3.7Observing Sound
3.3.8Sound waves are Longitudinal Waves
3.3.9Uses of Sound Waves
3.3.10The Interactions of Sound with Different Mediums
3.3.11Reflecting Sounds
3.3.12The Speed of Sound
3.3.13Measuring the Speed of Sound
3.3.14The Hearing Range of Humans
3.3.15The Human Ear
3.3.16Light Waves
3.3.17Coloured Light
3.3.18Observing Light
3.3.19Reflection
3.3.20Drawing a Reflected Image
3.3.21Refraction of Light 1
3.3.22Refraction of Light 2
3.3.23The Human Eye
3.3.24The Eye as a Pinhole Camera
3.3.25Lenses
3.3.26Colour
3.3.27Seeing Colour
3.3.28Colours of Light
3.3.29Drawing Waves
3.3.30Wave Interactions
3.3.31Comparing Sound & Light
3.3.32General Principles of Radiation
3.3.33Temperature & Black Bodies
3.3.34Production of Electromagnetic Waves
3.3.35Sound as Radiation
3.3.36Light as Radiation
3.3.37End of Topic Test - Sound
3.3.38End of Topic Test - Waves
3.3.39Diagnostic Misconceptions - Reflection
3.3.40Diagnostic Misconceptions - Wave Speed
3.4Electricity & Magnetism
3.4.1Circuit Symbols
3.4.2Resistors & Diodes
3.4.3Electric Current
3.4.4Measuring Current
3.4.5Potential Difference
3.4.6Series Circuits
3.4.7Parallel Circuits
3.4.8Resistance
3.4.9Charges
3.4.10Static Electricity
3.4.11Magnets
3.4.12Magnetic Fields
3.4.13The Earth's Field
3.4.14Electromagnetism
3.4.15Uses of Electromagnets
3.4.16Strength of Magnetic Fields
3.4.17Circuit Symbols HyperLearning
3.4.18Diagnostic Misconceptions - Positive Charge
3.4.19Diagnostic Misconceptions - Powering a Circuit
3.5Matter
3.5.1Physical Reactions
3.5.2Changes of State
3.5.3Particles
3.5.4Density
3.5.5Density & the Particle Model
3.5.6The Equation for Density
3.5.7Dissolving
3.5.8Brownian Motion
3.5.9Diffusion
3.5.10Filtration
3.5.11Solids
3.5.12Liquids
3.5.13Gases
3.5.14Weight & Mass
3.5.15Gravity
3.5.16Gravitational Field Strength
3.5.17Gravity in Space
3.5.18Atmospheric Pressure
3.5.19Liquid Pressure
3.5.20End of Topic Test - Matter
3.5.21Diagnostic Misconceptions - Floating
4Disciplinary Knowledge
4.1Thinking Scientifically
4.1.1Strengths & Limitations of Models
4.1.2Symbols & Formulae to Represent Scientific Ideas
4.1.3Analogies in Science
4.1.4Changing Models – Atomic Theory
4.1.5Working Safely in the Lab
4.1.6Variables
4.1.7Variables - Common Experiments
4.1.8Writing a Hypothesis & Prediction
4.1.9Planning an Experiment
4.1.10Maths Skills for Science - Mean
4.1.11Maths Skills for Science - Percentage & Equations
4.1.12Drawing Scientific Apparatus
4.1.13Observation & Measurement Skills
4.1.14Types of Data
4.1.15Graphs & Charts
4.1.16Bias in Science
4.1.17Conclude & Evaluate
4.2Applications of Science and its Implications
Jump to other topics
1Biology
1.1Cells, Tissues & Organs
1.1.1Microscopes
1.1.2Magnification
1.1.3Multicellular Organisms
1.1.4Tissues
1.1.5Organs
1.1.6Unicellular Organisms
1.1.7Diffusion
1.1.8Factors Affecting Diffusion
1.1.9Plant Cells
1.1.10Cellulose
1.1.11Plant Tissues
1.1.12Leaves
1.1.13Animal Cells
1.1.14Comparing Animal & Plant Cells
1.1.15How to Make a Model Animal and Plant Cell
1.1.16Specialised Cells
1.1.17Stem Cells
1.1.18Uses of Stem Cells
1.1.19Disadvantages of Stem Cells
1.1.20Blood Components
1.1.21Platelets
1.1.22End of Topic Test - Cells & Organisation
1.1.23The Lungs
1.1.24Breathing
1.1.25Plant Gas Exchange
1.1.26Drugs
1.1.27End of Topic Test - Living Organisms
1.1.28Diagnostic Misconceptions - Microbes
1.1.29Diagnostic Misconceptions - Diff. vs Specialised
1.1.30Diagnostic Misconceptions - Types of Cell
1.1.31Diagnostic Misconceptions - Plants & Animal Cells
1.2Reproduction & Variation
1.2.1Reproduction in Humans
1.2.2Male Reproductive System
1.2.3Female Reproductive System
1.2.4Gestation
1.2.5Pregnancy
1.2.6Puberty
1.2.7The Menstrual Cycle
1.2.8Reproduction in Plants
1.2.9Asexual Reproduction
1.2.10Advantages of Asexual Reproduction
1.2.11Adaptations
1.2.12Evolution
1.2.13Pollination
1.2.14Dispersal Method
1.2.15Variation
1.2.16Causes of Variation
1.2.17Inheritance
1.2.18Species & Selective Breeding
1.2.19Genetic Conditions - Cystic Fibrosis
1.2.20Genetic Conditions - Haemophilia
1.2.21End of Topic Test - Reproduction & Variation
1.2.22Diagnostic Misconceptions - Asexual
1.3Ecological Relationships & Classification
1.3.1Species Interdependence
1.3.2Food Chains & Webs
1.3.3Changes to Food Webs - Plants
1.3.4Changes to Food Webs - Animals
1.3.5Changes to Food Webs - Factors
1.3.6Relationships in an Ecosystem
1.3.7The Impact of Environmental Change
1.3.8Decomposers
1.3.9Decay
1.3.10Assessing Ecosystems
1.3.11Ecological Sampling - Quadrats
1.3.12Ecological Sampling
1.3.13Required Practical - Estimating Population Size
1.3.14Pyramids of Number and Biomass
1.3.15Classification of Living Organisms
1.3.16Competition Between Organisms
1.3.17Adaptations of Plants
1.3.18Natural Selection
1.3.19Evidence for Evolution
1.3.20Environmental Changes & Extinctions
1.3.21The Importance of Biodiversity
1.3.22Bioaccumulation
1.3.23Bioaccumulation - DDT and Mercury
1.3.24End of Topic Test - Material Cycles & Energy
1.4Digestion & Nutrition
1.4.1Balanced Diets
1.4.2Vitamins & Minerals
1.4.3Protein
1.4.4Lipids, Oils and Fats
1.4.5Carbohydrates
1.4.6Starch
1.4.7Energy Needs
1.4.8Dietary Fibre
1.4.9Diseases Caused by Nutritional Deficiencies
1.4.10Digestion
1.4.11Plant Nutrition
1.4.12Enzymes in Digestion
1.4.13Required Practical - Enzymes in Digestion
1.4.14Diagnostic Misconceptions - Digestion
1.4.15Diagnostic Misconceptions - Excretion vs Egestion
1.5Plants & Photosynthesis
1.5.1Roots
1.5.2Photosynthesis
1.5.3Leaves in Photosynthesis
1.5.4Rate of Photosynthesis
1.5.5Testing the Rate of Photosynthesis
1.5.6Water Transport in Plants
1.5.7Translocation
1.5.8The Carbon Cycle
1.5.9Human Activities & Carbon Dioxide
1.5.10Diagnostic Misconceptions - Plant Food
1.5.11Diagnostic Misconceptions - Transpiration
1.6Biological Systems & Processes
1.6.1Living Organisms
1.6.2Dichotomous Keys
1.6.3Biomechanics
1.6.4Muscles
1.6.5The Skeleton
1.6.6Bones
1.6.7Tendons and Ligaments
1.6.8Carpals and Tarsals
1.6.9Measuring Forces
1.6.10Antagonistic Muscle Pairings
1.6.11The Respiratory System
1.6.12Structure & Function of the Gas Exchange System
1.6.13Breathing
1.6.14Respiration
1.6.15Respiration During Exercise
1.6.16Anaerobic Respiration
1.6.17Lactic Acid
1.6.18Effects of Smoking on the Respiratory System
1.6.19Balanced Diets
1.6.20Human Growth & Development
1.6.21DNA
1.6.22Alleles
1.6.23Genotype vs Phenotype
1.6.24Punnett Squares
1.6.25Joints
1.6.26The Renal System
1.6.27The Double Circulatory System
1.6.28Gas Exchange
1.6.29Heart and Blood
1.6.30Blood Vessels
1.6.31Glucose
1.6.32Glucose and Diabetes
1.6.33The Effects of Recreational Drug Use
1.6.34Human Illnesses
1.6.35Bacterial and Viral Infections
1.6.36Antibiotics
1.6.37Vaccinations
1.6.38How Antibiotics and Vaccines Work
1.6.39Mental Health
1.6.40Depression and Anxiety
2Chemistry
2.1Particles
2.1.1Particles
2.1.2States of Matter
2.1.3Compression of Substances
2.1.4Shape of Substances
2.1.5Changes of State
2.1.6Elements and Atoms
2.1.7Compounds
2.1.8Diffusion
2.1.9Changing State
2.1.10Pressure
2.1.11Temperature Increase in a Gas
2.1.12Conservation of Mass
2.1.13Purity of Substances
2.1.14Pure Substances
2.1.15Evaporation
2.1.16Mixtures
2.1.17Separating Mixtures
2.1.18Distillation
2.1.19Chromatography
2.1.20Solubility
2.1.21Investigating Solubility
2.1.22Chemical Symbols
2.1.23Chemical Formulae
2.1.24Ceramics
2.1.25Polymers
2.1.26Composites
2.1.27Diagnostic Misconceptions - Particles
2.1.28Diagnostic Misconceptions - Temperature
2.1.29Diagnostic Misconceptions - Boiling Liquids
2.2Chemical Reactions
2.3Atoms, Elements, Compounds
2.4The Periodic Table
2.5Materials & the Earth
2.5.1The Composition of The Earth
2.5.2The Structure of the Earth
2.5.3Igneous Rocks
2.5.4Sedimentary Rocks
2.5.5Metamorphic Rocks
2.5.6The Rock Cycle
2.5.7Physical Weathering
2.5.8Chemical Weathering
2.5.9Biological Weathering
2.5.10The Formation of Fossils
2.5.11Crude Oil
2.5.12End of Topic Test - Earth
2.5.13The Earth's Early Atmosphere
2.5.14The Earth's Atmosphere Today
2.5.15Oxygen in the Atmosphere
2.5.16Carbon Dioxide in the Atmosphere
2.5.17Greenhouse Gases
2.5.18Climate Change
2.5.19Resources
2.5.20Recycling
2.5.21Ceramics
2.5.22Polymers
2.5.23Composites
2.5.24End of Topic Test - Materials
2.5.25End of Topic Test - Polymers
2.5.26Diagnostic Misconceptions - Air
2.5.27Diagnostic Misconceptions - Greenhouse Effect
2.6Reactivity
2.6.1Ions
2.6.2Ionic Bonding
2.6.3State Symbols
2.6.4Balancing Chemical Equations
2.6.5Relative Formula Mass
2.6.6Calculating the Relative Formula Mass
2.6.7The Reactivity Series
2.6.8Carbon & The Reactivity Series
2.6.9Displacement Reactions
2.6.10Displacement Reactions - Halogens
2.6.11Alloys
2.6.12Metal Alloys
2.7Energetics
2.8Properties of Materials
3Physics
3.1Energy
3.1.1Energy Stores & Pathways
3.1.2Energy Transfers
3.1.3Common Energy Transfers
3.1.4Wasted Energy
3.1.5Efficiency of Energy Transfer
3.1.6Sankey Diagrams
3.1.7Heat & Temperature
3.1.8Heat Transfer
3.1.9Conductors vs Insulators
3.1.10Reducing Energy Transfers
3.1.11Energy & Power
3.1.12Energy in Food
3.1.13Calories
3.1.14Food Labels
3.1.15Power Ratings of Appliances
3.1.16Comparing Bulbs
3.1.17Fuel Bills
3.1.18Calculating Fuel Bills
3.1.19Non-Renewable Energy - Fossil Fuels
3.1.20Other Non-Renewables
3.1.21Renewable Energy - Air & Ground
3.1.22Renewable Energy - Water
3.1.23Conservation of Energy
3.1.24End of Topic Test - Energy
3.1.25Diagnostic Misconceptions - Stationary Objects
3.2Forces & Motion
3.2.1Forces
3.2.2Contact Forces
3.2.3Balanced Forces
3.2.4Force Diagrams & Resultant Forces
3.2.5Free Body Diagram - Uses
3.2.6Force & Acceleration
3.2.7Gravity
3.2.8Weight
3.2.9Pressure
3.2.10Speed
3.2.11Relative Motion
3.2.12Friction 1
3.2.13Friction 2
3.2.14Turning Effect
3.2.15Moment
3.2.16Equation for Moments
3.2.17Water & Air Resistance
3.2.18Distance-Time Graphs
3.2.19Levers
3.2.20Work & Machines
3.2.21Work
3.2.22Machines
3.2.23Elasticity
3.2.24Elasticity - Hooke's Law
3.2.25Archimedes Principle
3.2.26Floating & Sinking
3.2.27End of Topic Test - Forces & Motion
3.2.28Vacuums
3.2.29Thermal Energy & Conduction
3.2.30Convection & Radiation
3.2.31Evaporation
3.2.32Diagnostic Misconceptions - Weight vs Mass
3.2.33Diagnostic Misconceptions - Gravity
3.3Waves
3.3.1Waves
3.3.2Types of Waves
3.3.3Observing Waves
3.3.4Wave Speed
3.3.5Earthquakes
3.3.6Hearing
3.3.7Observing Sound
3.3.8Sound waves are Longitudinal Waves
3.3.9Uses of Sound Waves
3.3.10The Interactions of Sound with Different Mediums
3.3.11Reflecting Sounds
3.3.12The Speed of Sound
3.3.13Measuring the Speed of Sound
3.3.14The Hearing Range of Humans
3.3.15The Human Ear
3.3.16Light Waves
3.3.17Coloured Light
3.3.18Observing Light
3.3.19Reflection
3.3.20Drawing a Reflected Image
3.3.21Refraction of Light 1
3.3.22Refraction of Light 2
3.3.23The Human Eye
3.3.24The Eye as a Pinhole Camera
3.3.25Lenses
3.3.26Colour
3.3.27Seeing Colour
3.3.28Colours of Light
3.3.29Drawing Waves
3.3.30Wave Interactions
3.3.31Comparing Sound & Light
3.3.32General Principles of Radiation
3.3.33Temperature & Black Bodies
3.3.34Production of Electromagnetic Waves
3.3.35Sound as Radiation
3.3.36Light as Radiation
3.3.37End of Topic Test - Sound
3.3.38End of Topic Test - Waves
3.3.39Diagnostic Misconceptions - Reflection
3.3.40Diagnostic Misconceptions - Wave Speed
3.4Electricity & Magnetism
3.4.1Circuit Symbols
3.4.2Resistors & Diodes
3.4.3Electric Current
3.4.4Measuring Current
3.4.5Potential Difference
3.4.6Series Circuits
3.4.7Parallel Circuits
3.4.8Resistance
3.4.9Charges
3.4.10Static Electricity
3.4.11Magnets
3.4.12Magnetic Fields
3.4.13The Earth's Field
3.4.14Electromagnetism
3.4.15Uses of Electromagnets
3.4.16Strength of Magnetic Fields
3.4.17Circuit Symbols HyperLearning
3.4.18Diagnostic Misconceptions - Positive Charge
3.4.19Diagnostic Misconceptions - Powering a Circuit
3.5Matter
3.5.1Physical Reactions
3.5.2Changes of State
3.5.3Particles
3.5.4Density
3.5.5Density & the Particle Model
3.5.6The Equation for Density
3.5.7Dissolving
3.5.8Brownian Motion
3.5.9Diffusion
3.5.10Filtration
3.5.11Solids
3.5.12Liquids
3.5.13Gases
3.5.14Weight & Mass
3.5.15Gravity
3.5.16Gravitational Field Strength
3.5.17Gravity in Space
3.5.18Atmospheric Pressure
3.5.19Liquid Pressure
3.5.20End of Topic Test - Matter
3.5.21Diagnostic Misconceptions - Floating
4Disciplinary Knowledge
4.1Thinking Scientifically
4.1.1Strengths & Limitations of Models
4.1.2Symbols & Formulae to Represent Scientific Ideas
4.1.3Analogies in Science
4.1.4Changing Models – Atomic Theory
4.1.5Working Safely in the Lab
4.1.6Variables
4.1.7Variables - Common Experiments
4.1.8Writing a Hypothesis & Prediction
4.1.9Planning an Experiment
4.1.10Maths Skills for Science - Mean
4.1.11Maths Skills for Science - Percentage & Equations
4.1.12Drawing Scientific Apparatus
4.1.13Observation & Measurement Skills
4.1.14Types of Data
4.1.15Graphs & Charts
4.1.16Bias in Science
4.1.17Conclude & Evaluate
4.2Applications of Science and its Implications
Unlock your full potential with Seneca Premium
Unlimited access to 10,000+ open-ended exam questions
Mini-mock exams based on your study history
Unlock 800+ premium courses & e-books