3.2.7
Reactions of Alkenes
Alkenes
Alkenes
Alkenes are molecules with a double covalent bond. There is a high electron density between the carbon atoms because of the double bond.


Reactions of alkenes
Reactions of alkenes
- Alkenes react with several reagents in addition reactions.
- The alkenes are nucleophiles because of the high electron density between the carbon atoms.
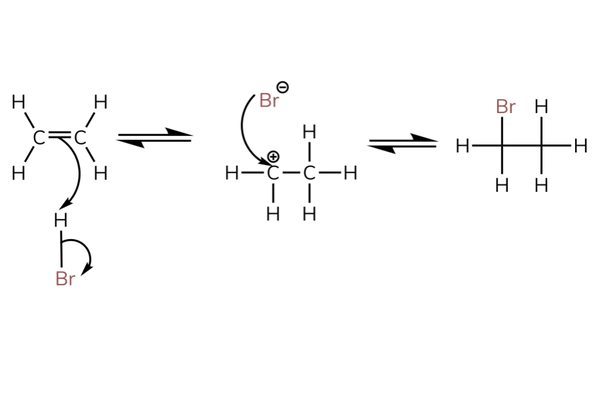
- The mechanism is shown next for the reaction with hydrogen bromide.
- The mechanism is called the electrophilic addition mechanism (because an electrophile is added).
- They will react in a similar way with sulfuric acid, and bromine.

Electrophilic addition
Electrophilic addition
- Above shows the reaction with HBr.
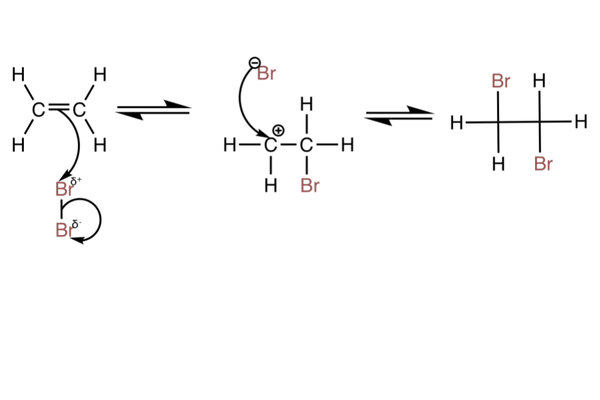

With bromine
With bromine
- The reaction can also be done with Br2.
- The partial charges are assigned to the bromine atoms because the high electron density of the double bond repels the bonding electrons and polarises the bond - this is shown in the mechanism.
Uses of Electrophilic Addition
Uses of Electrophilic Addition
Electrophilic addition reactions between alkenes and bromine as well as alkenes and sulfuric acid are useful.


With bromine
With bromine
- The reaction with bromine is used as a test for alkenes.
- Bromine water is a light orange colour and will be decolourised in the presence of an alkene (light orange → colourless).
- This test works because bromine can add to alkenes via an electrophilic addition reaction.


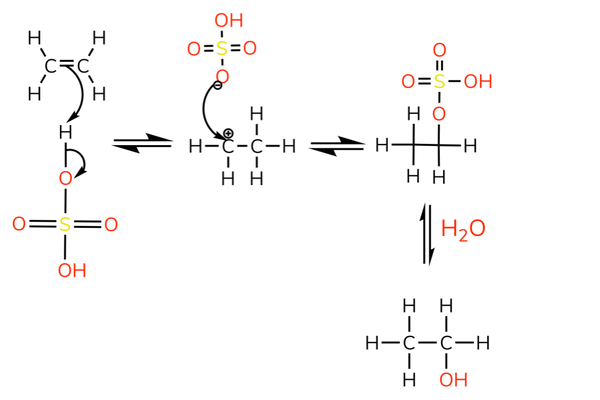
With sulfuric acid
With sulfuric acid
- The reaction with sulfuric acid is used to produce alcohols.
- The sulfate ion is removed by nucleophilic substitution.
Asymmetric Electrophilic Addition
Asymmetric Electrophilic Addition
Asymmetric alkenes can have multiple products in electrophilic addition reactions.
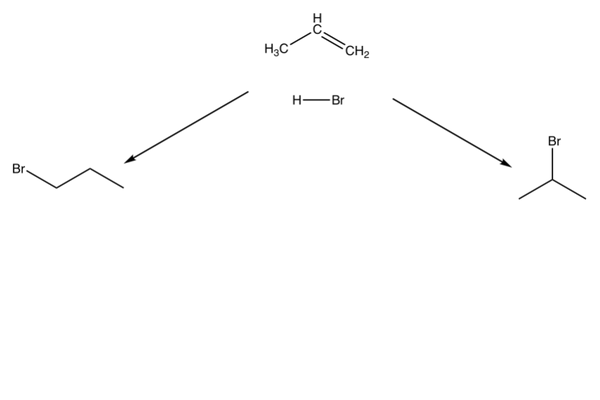

Multiple products
Multiple products
- If you have an asymmetric alkene, you can get multiple products.
- A reaction will favour one of the products over the other - we call this selectivity.
- This is shown for the reaction of propene with HBr.
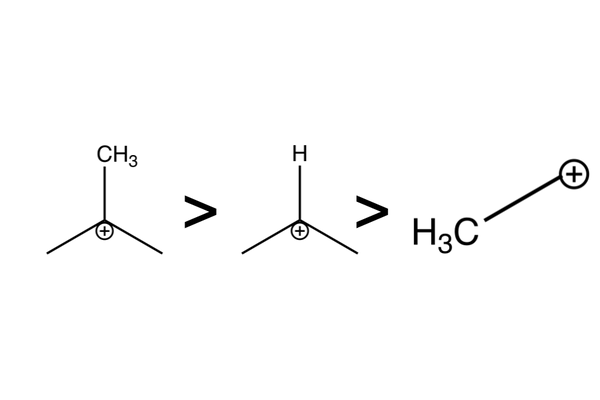

Intermediate stability
Intermediate stability
- This selectivity is driven by carbocation intermediate stability:
- Tertiary carbocations > secondary carbocations > primary carbocations.
- The more alkyl groups there are next to the positive charge, the more stable the intermediate is.
- Alkyl groups push electrons and so help to stabilise adjacent positive charge.


Which product when?
Which product when?
- When considering which product is most likely, we must consider the intermediates formed.
- 2-bromopropane is the most likely product because the intermediate is a secondary carbocation, rather than a primary carbocation.
- This is because the secondary carbocation is more stable.
- It has more alkyl groups pushing electrons onto the positive charge.
- This is because the secondary carbocation is more stable.
- Both reactions will happen and you will get both products, but you will have one major and one minor.
1Physical Chemistry
1.1Atoms, Molecules & Stoichiometry
1.2Atomic Structure
1.2.1Fundamental Particles
1.2.2Isotopes & Mass Number
1.2.3Electron Shells, Sub-Shells & Orbitals
1.2.4Electron Configuration
1.2.5Ionisation Energy
1.2.6Factors Affecting Ionisation Energies
1.2.7Trends of Ionisation
1.2.8Specific Impacts on Ionisation Energies
1.2.9Electron Affinity
1.2.10End of Topic Test - Atomic Structure
1.2.11A-A* (AO2/3) - Atomic Structure
1.3Chemical Bonding
1.3.1Ionic Bonding
1.3.2Covalent & Dative Bonding
1.3.3Shapes of Molecules
1.3.4Intermolecular Forces
1.3.5Intermolecular Forces 2
1.3.6Electronegativity
1.3.7Bond Length, Bond Energy, & Bond Polarity
1.3.8Metallic Bonding
1.3.9Physical Properties
1.3.10End of Topic Test - Bonding
1.3.11A-A* (AO2/3) - Bonding
1.4States of Matter
1.5Chemical Energetics
1.6Electrochemistry
1.7Equilibria
1.7.1Dynamic Equilibrium & Le Chatelier
1.7.2Kc
1.7.3Kp
1.7.4pH
1.7.5The Ionic Product of Water
1.7.6Weak Acids & Bases
1.7.7Introduction to Solubility Equilibria
1.7.8Solubility Equilibria Calculations
1.7.9Free Energy of Dissolution
1.7.10pH and Solubility
1.7.11Common-Ion Effect
1.7.12End of Topic Test - Kp & Electrochemistry
1.7.13A-A* (AO2/3) - Electrochemical Cells
1.8Partition Coefficient
1.9Reaction Kinetics
1.9.1Collision Theory
1.9.2Orders, Rate Constants & Equations
1.9.3Rate Graphs
1.9.4Rate Determining Step
1.9.5Maxwell-Boltzmann Distribution
1.9.6Catalysts
1.9.7Homogeneous Catalysts
1.9.8Heterogeneous Catalysts
1.9.9End of Topic Test - Kinetics
1.9.10End of Topic Test - Rate Equations
1.9.11A-A* (AO2/3) - Rate Equations
2Inorganic Chemistry
2.1The Periodic Table
2.2Group 2
2.3Group 17
2.4Transition Metals
3Organic Chemistry & Analysis
3.1Introduction to Organic Chemistry
3.2Hydrocarbons
3.2.1Fractional Distillation
3.2.2Cracking
3.2.3Combustion
3.2.4Chlorination
3.2.5End of Topic Test - Alkanes
3.2.6Introduction to Alkenes
3.2.7Reactions of Alkenes
3.2.8Polymerisation Reactions
3.2.9End of Topic Test - Alkenes
3.2.10Arenes
3.2.11Evidence for Structure of Arenes
3.2.12Reactions of Benzene
3.2.13End of Topic Test -Arenes
3.3Halogen Derivatives
3.4Hydroxy Compounds
3.5Carbonyl Compounds
3.6Carboxylic Acids & Derivatives
3.7Nitrogen Compounds
3.8Polymerisation
3.9Analytical Techniques
3.9.1Chromatography
3.9.2High-Performance Liquid Chromatography
3.9.3Gas Chromatography
3.9.4IR Spectroscopy
3.9.5Uses of IR Spectroscopy
3.9.6Mass Spectrometry
3.9.7Mass Spectrometry Analysis
3.9.8Nuclear Magnetic Resonance
3.9.9Carbon-13 NMR
3.9.10Proton NMR I
3.9.11Proton NMR II
3.9.12End of Topic Test - Analytical Techniques
3.9.13A-A* (AO2/3) - Analytical Techniques
Jump to other topics
1Physical Chemistry
1.1Atoms, Molecules & Stoichiometry
1.2Atomic Structure
1.2.1Fundamental Particles
1.2.2Isotopes & Mass Number
1.2.3Electron Shells, Sub-Shells & Orbitals
1.2.4Electron Configuration
1.2.5Ionisation Energy
1.2.6Factors Affecting Ionisation Energies
1.2.7Trends of Ionisation
1.2.8Specific Impacts on Ionisation Energies
1.2.9Electron Affinity
1.2.10End of Topic Test - Atomic Structure
1.2.11A-A* (AO2/3) - Atomic Structure
1.3Chemical Bonding
1.3.1Ionic Bonding
1.3.2Covalent & Dative Bonding
1.3.3Shapes of Molecules
1.3.4Intermolecular Forces
1.3.5Intermolecular Forces 2
1.3.6Electronegativity
1.3.7Bond Length, Bond Energy, & Bond Polarity
1.3.8Metallic Bonding
1.3.9Physical Properties
1.3.10End of Topic Test - Bonding
1.3.11A-A* (AO2/3) - Bonding
1.4States of Matter
1.5Chemical Energetics
1.6Electrochemistry
1.7Equilibria
1.7.1Dynamic Equilibrium & Le Chatelier
1.7.2Kc
1.7.3Kp
1.7.4pH
1.7.5The Ionic Product of Water
1.7.6Weak Acids & Bases
1.7.7Introduction to Solubility Equilibria
1.7.8Solubility Equilibria Calculations
1.7.9Free Energy of Dissolution
1.7.10pH and Solubility
1.7.11Common-Ion Effect
1.7.12End of Topic Test - Kp & Electrochemistry
1.7.13A-A* (AO2/3) - Electrochemical Cells
1.8Partition Coefficient
1.9Reaction Kinetics
1.9.1Collision Theory
1.9.2Orders, Rate Constants & Equations
1.9.3Rate Graphs
1.9.4Rate Determining Step
1.9.5Maxwell-Boltzmann Distribution
1.9.6Catalysts
1.9.7Homogeneous Catalysts
1.9.8Heterogeneous Catalysts
1.9.9End of Topic Test - Kinetics
1.9.10End of Topic Test - Rate Equations
1.9.11A-A* (AO2/3) - Rate Equations
2Inorganic Chemistry
2.1The Periodic Table
2.2Group 2
2.3Group 17
2.4Transition Metals
3Organic Chemistry & Analysis
3.1Introduction to Organic Chemistry
3.2Hydrocarbons
3.2.1Fractional Distillation
3.2.2Cracking
3.2.3Combustion
3.2.4Chlorination
3.2.5End of Topic Test - Alkanes
3.2.6Introduction to Alkenes
3.2.7Reactions of Alkenes
3.2.8Polymerisation Reactions
3.2.9End of Topic Test - Alkenes
3.2.10Arenes
3.2.11Evidence for Structure of Arenes
3.2.12Reactions of Benzene
3.2.13End of Topic Test -Arenes
3.3Halogen Derivatives
3.4Hydroxy Compounds
3.5Carbonyl Compounds
3.6Carboxylic Acids & Derivatives
3.7Nitrogen Compounds
3.8Polymerisation
3.9Analytical Techniques
3.9.1Chromatography
3.9.2High-Performance Liquid Chromatography
3.9.3Gas Chromatography
3.9.4IR Spectroscopy
3.9.5Uses of IR Spectroscopy
3.9.6Mass Spectrometry
3.9.7Mass Spectrometry Analysis
3.9.8Nuclear Magnetic Resonance
3.9.9Carbon-13 NMR
3.9.10Proton NMR I
3.9.11Proton NMR II
3.9.12End of Topic Test - Analytical Techniques
3.9.13A-A* (AO2/3) - Analytical Techniques
Unlock your full potential with Seneca Premium
Unlimited access to 10,000+ open-ended exam questions
Mini-mock exams based on your study history
Unlock 800+ premium courses & e-books