13.1.5
Investigating Photosynthesis
Investigating Dehydrogenase Activity in Chloroplasts
Investigating Dehydrogenase Activity in Chloroplasts
During the light-dependent reaction, NADP is reduced to NADPH. A dehydrogenase enzyme catalyses this reaction. This experiment will monitor dehydrogenase activity by using DCPIP, a redox indicator.
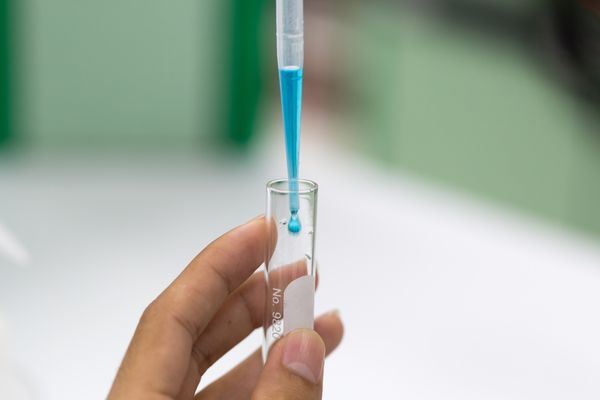

DCPIP
DCPIP
- In this investigation, a blue dye (DCPIP) is used to monitor the rate of dehydrogenase activity. DCPIP is a redox indicator.
- This means that it is blue in the oxidised state and colourless in its reduced state.
- When electrons are released by the chlorophyll, DCPIP will change from blue to colourless.


1) Extracting chloroplasts
1) Extracting chloroplasts
- Put 50 cm3 of isolation medium into a beaker.
- Tear eight spinach leaves into small pieces and put the pieces into the isolation medium in the beaker.
- Do NOT put pieces of the midrib or the leaf stalk into the beaker.
- Half fill a large beaker with ice and place a small beaker on top of the ice.
- When carrying out this step, all solutions and apparatus should be kept as cold as possible and the extraction should be carried out as quickly as possible.


2) Suspending chloroplasts
2) Suspending chloroplasts
- Put 3 layers of muslin over the top of the filter funnel and wet with the isolation medium. Rest the filter funnel in the small beaker on the ice.
- Pour the spinach and isolation medium into the blender and blend for 15 seconds. Pour the blended mixture back into the beaker.
- Pour your blended mixture through the muslin in the filter funnel. Carefully squeeze the muslin to assist the filtering process.
- Label this filtrate which is in the small beaker on ice as ‘chloroplast suspension’.


4) Set up tubes A and B
4) Set up tubes A and B
- Label five test tubes A, B, C, X and Y and stand them in the large beaker. Put the lamp about 10 cm away so that all tubes are illuminated. Set up tubes A and B as follows:
- Tube A - 5 cm3 DCPIP solution + 1 cm3 water + 1 cm3 chloroplast suspension. Immediately wrap the tube in aluminium foil to exclude light.
- Tube B - 5 cm3 DCPIP solution + 1 cm3 water + 1 cm3 isolation medium.
- Tubes A and B are control experiments. Leave both tubes until the end of your investigation.


5) Set up tube C
5) Set up tube C
- Set up tube C as follows:
- Tube C - 6 cm3 water + 1 cm3 chloroplast suspension.
- Tube C is for you to use as a standard to help you to determine when any colour change is complete.


6) Set up tube X
6) Set up tube X
- Set up tube X as follows:
- Tube X - 5 cm3 DCPIP solution + 1 cm3 water in the tube.
- Add 1 cm3 chloroplast suspension to tube X, quickly mix the contents and start the timer.
- Record in seconds how long it takes for the contents of tube X to change colour from blue-green to green. Use tube C to help you determine when the colour change is complete.
- Repeat this step four more times.


7) Set up tube Y
7) Set up tube Y
- Set up tube Y as follows:
- Tube Y - 5 cm3 DCPIP solution + 1 cm3 ammonium hydroxide.
- Add 1 cm3 chloroplast suspension to tube Y, quickly mix the contents and start the timer.
- Record in seconds how long it takes for the contents of tube Y to change colour from blue-green to green. Use tube C to help you determine when the colour change is complete.
- Repeat this step four more times.


8) Record the results
8) Record the results
- Record your data in a suitable table.
- At the end of your investigation, record the colour of the mixtures in tubes A and B.
The Hill Reaction
The Hill Reaction
The Hill reaction was named after Robert Hill, who designed this experiment to understand more about the process of photosynthesis. It investigates the light-dependent reaction in photosynthesis.


The Hill Reaction
The Hill Reaction
- The Hill reaction follows these generalised steps:
- Extract chloroplasts from a plant.
- Suspend chloroplasts in solution.
- Add DCPIP (an electron acceptor in place of NADP), which is used to observe whether redox reactions are taking place, to the treatment solutions and a control solution.
- Expose chloroplast solutions to light and record any colour changes of DCPIP.


DCPIP
DCPIP
- DCPIP is an electron acceptor which acts as a substitute for NADP in this reaction.
- DCPIP is blue when oxidised, and colourless when reduced.
- If DCPIP turns from blue to colourless, this means it is accepting electrons and a redox reaction is taking place.
 4.2.4.1 - The pH scale,h_400,q_80,w_640.png)
 4.2.4.1 - The pH scale,h_400,q_80,w_640.png)
Possible reactions to investigate
Possible reactions to investigate
- The Hill reaction can be used to investigate the effect of different variables on photosynthesis.
- Solutions can be heated to different temperatures to investigate how temperature affects rate of photosynthesis.
- Solutions can be exposed to light or dark to see how light intensity affects rate of photosynthesis.
- Solutions can be made up to different pH levels to investigate effect of pH on rate of photosynthesis.
- The speed at which DCPIP changes from blue to colourless is inversely related to rate of photosynthesis.


Important findings
Important findings
- Hill found that when isolated chloroplasts were exposed to light, the added DCPIP turned from blue to colourless, but not when the chloroplasts were kept in the dark.
- This was an important finding because it showed that photosynthesis depends at least partly on light, and that redox reactions were happening.
- The reaction also showed that light was needed for oxygen to be produced, and that production of oxygen happens in a different step to fixation of carbon dioxide.
1Cell Structure
1.1Cell Structure
1.1.1Studying Cells - Microscopes
1.1.2Introduction to Eukaryotic & Prokaryotic Cells
1.1.3Ultrastructure of Eukaryotic Cells
1.1.4Ultrastructure of Eukaryotic Cells 2
1.1.5Ultrastructure of Eukaryotic Cells 3
1.1.6Prokaryotic Cells
1.1.7Viruses
1.1.8End of Topic Test - Cell Structure
1.1.9Exam-Style Question - Microscopes
1.1.10A-A* (AO2/3) - Cell Structure
2Biological Molecules
2.1Testing for Biological Modules
2.2Carbohydrates & Lipids
2.3Proteins
3Enzymes
4Cell Membranes & Transport
4.1Biological Membranes
5The Mitotic Cell Cycle
6Nucleic Acids & Protein Synthesis
6.1Nucleic Acids
7Transport in Plants
8Transport in Mammals
8.1Circulatory System
8.2Transport of Oxygen & Carbon Dioxide
9Gas Exchange
9.1Gas Exchange System
10Infectious Diseases
10.1Infectious Diseases
10.2Antibiotics
11Immunity
12Energy & Respiration (A2 Only)
13Photosynthesis (A2 Only)
14Homeostasis (A2 Only)
14.1Homeostasis
14.2The Kidney
14.3Cell Signalling
14.4Blood Glucose Concentration
14.5Homeostasis in Plants
15Control & Coordination (A2 Only)
15.1Control & Coordination in Mammals
15.1.1Neurones
15.1.2Receptors
15.1.3Taste
15.1.4Reflexes
15.1.5Action Potentials
15.1.6Saltatory Conduction
15.1.7Synapses
15.1.8Cholinergic Synnapses
15.1.9Neuromuscular Junction
15.1.10Skeletal Muscle
15.1.11Sliding Filament Theory Contraction
15.1.12Sliding Filament Theory Contraction 2
15.1.13Menstruation
15.1.14Contraceptive Pill
15.2Control & Co-Ordination in Plants
16Inherited Change (A2 Only)
16.1Passage of Information to Offspring
16.2Genes & Phenotype
17Selection & Evolution (A2 Only)
17.2Natural & Artificial Selection
18Classification & Conservation (A2 Only)
18.1Biodiversity
18.2Classification
19Genetic Technology (A2 Only)
19.1Manipulating Genomes
19.2Genetic Technology Applied to Medicine
19.3Genetically Modified Organisms in Agriculture
Jump to other topics
1Cell Structure
1.1Cell Structure
1.1.1Studying Cells - Microscopes
1.1.2Introduction to Eukaryotic & Prokaryotic Cells
1.1.3Ultrastructure of Eukaryotic Cells
1.1.4Ultrastructure of Eukaryotic Cells 2
1.1.5Ultrastructure of Eukaryotic Cells 3
1.1.6Prokaryotic Cells
1.1.7Viruses
1.1.8End of Topic Test - Cell Structure
1.1.9Exam-Style Question - Microscopes
1.1.10A-A* (AO2/3) - Cell Structure
2Biological Molecules
2.1Testing for Biological Modules
2.2Carbohydrates & Lipids
2.3Proteins
3Enzymes
4Cell Membranes & Transport
4.1Biological Membranes
5The Mitotic Cell Cycle
6Nucleic Acids & Protein Synthesis
6.1Nucleic Acids
7Transport in Plants
8Transport in Mammals
8.1Circulatory System
8.2Transport of Oxygen & Carbon Dioxide
9Gas Exchange
9.1Gas Exchange System
10Infectious Diseases
10.1Infectious Diseases
10.2Antibiotics
11Immunity
12Energy & Respiration (A2 Only)
13Photosynthesis (A2 Only)
14Homeostasis (A2 Only)
14.1Homeostasis
14.2The Kidney
14.3Cell Signalling
14.4Blood Glucose Concentration
14.5Homeostasis in Plants
15Control & Coordination (A2 Only)
15.1Control & Coordination in Mammals
15.1.1Neurones
15.1.2Receptors
15.1.3Taste
15.1.4Reflexes
15.1.5Action Potentials
15.1.6Saltatory Conduction
15.1.7Synapses
15.1.8Cholinergic Synnapses
15.1.9Neuromuscular Junction
15.1.10Skeletal Muscle
15.1.11Sliding Filament Theory Contraction
15.1.12Sliding Filament Theory Contraction 2
15.1.13Menstruation
15.1.14Contraceptive Pill
15.2Control & Co-Ordination in Plants
16Inherited Change (A2 Only)
16.1Passage of Information to Offspring
16.2Genes & Phenotype
17Selection & Evolution (A2 Only)
17.2Natural & Artificial Selection
18Classification & Conservation (A2 Only)
18.1Biodiversity
18.2Classification
19Genetic Technology (A2 Only)
19.1Manipulating Genomes
19.2Genetic Technology Applied to Medicine
19.3Genetically Modified Organisms in Agriculture
Unlock your full potential with Seneca Premium
Unlimited access to 10,000+ open-ended exam questions
Mini-mock exams based on your study history
Unlock 800+ premium courses & e-books