Back
Atomic Structure
Atomic Model
An atom has a small, positively-charged nucleus surrounded by orbiting negatively-charged electrons.
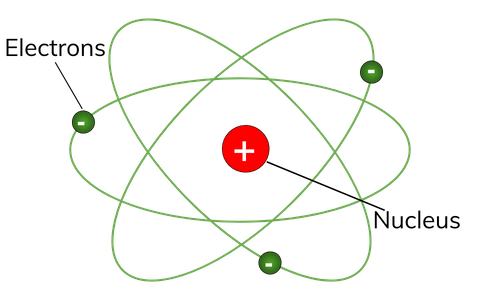
An atom has a small, positively-charged nucleus surrounded by orbiting negatively-charged electrons.
Structure of an Atom
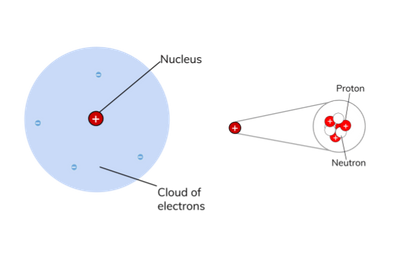
Atoms are very small and have a radius of about 1 × 10 ^ -10 metres. The electrons are arranged at different distances from the nucleus (different energy levels). The radius of a nucleus is less than 1/10 000 of the radius of an atom. Most of the mass of an atom is concentrated in the nucleus.
Want to learn more about Atomic Structure?
Join Seneca to get 250+ free exam board specfic A Level, GCSE, KS3 & KS2 online courses.





