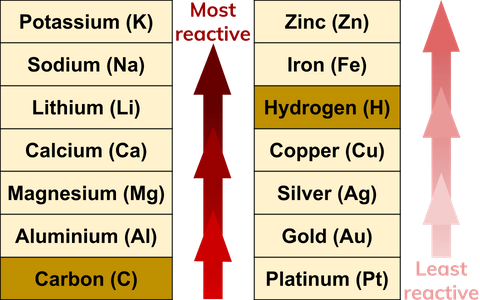
Reactivity Series
When metals react with other substances, the metal atoms always form positive ions. The easier it is for a metal to form its positive ion, the more reactive the metal is. Metals can be arranged in order of their reactivity. Very unreactive metals, such as gold and platinum, are found in the Earth’s crust as pure metals. These are called native metals.
Displacement Reactions
A more reactive metal (one that forms positive ions more easily) can displace a less reactive metal from a compound. This is called a displacement reaction.
Displacement order
A metal can only displace another metal from a compound if it is located above it in the reactivity series. A displacement reaction happens when a more reactive metal (one that forms positive ions more easily) displaces a less reactive metal from a compound.
Example:
Magnesium is more reactive than copper, so magnesium can displace copper from a copper sulfate solution to create magnesium sulfate.
Magnesium + copper sulfate → magnesium sulfate + copper
Mg(s) + CuSO4(aq) → MgSO4(aq) + Cu(s)
Platinum, however, is less reactive than copper and so cannot displace copper from a copper sulfate solution.
Reactivity Tests & Extraction
Potassium, sodium and lithium all react quickly with cold water to produce a metal hydroxide and hydrogen gas.
Most metals react with dilute acids to produce a salt and hydrogen gas.
Extraction of metals
Most metals are only found as compounds because the metal has reacted with other elements in the past. Because of this, the metal has to be extracted from the ore (rock) where the metal compound is found.
Want to learn more about Reactivity Series?
Join Seneca to get 250+ free exam board specfic A Level, GCSE, KS3 & KS2 online courses.





