Osmosis
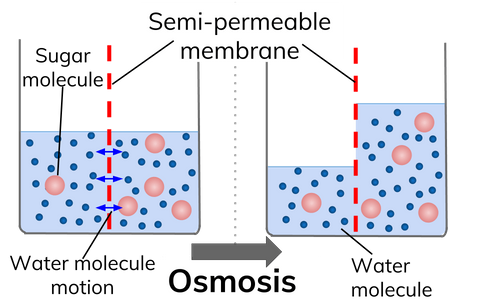
Osmosis is the diffusion of water across a partially permeable membrane from a dilute solution (high concentration of water) to a concentrated solution (low concentration of water).
In the diagram, the concentration of sugar is initially higher on the right side of the membrane. Therefore, water moves by osmosis to the right hand side to equalise the concentrations.
Partially permeable membrane
A partially permeable membrane allows water through, but won't let larger molecules dissolved in water pass through.
Water movement
Water will move to make the concentrations the same on both sides of the membrane: When there are lots of water molecules (in a dilute solution) on one side of a partially permeable membrane, but not many on the other side (in a concentrated solution), water will move from the dilute to the concentrated solution.
Net movement of water
It is important to remember that water molecules will move through the membrane in both directions, but the net (overall) movement of water will be from the dilute solution to a concentrated solution.
Want to learn more about Osmosis?
Join Seneca to get 250+ free exam board specfic A Level, GCSE, KS3 & KS2 online courses.





