Periodic Table
The periodic table is an ordered arrangement of all 118 known elements. The elements are arranged in order of their atomic number (number of protons). Every time you move an element to the right, the proton number increases by 1.
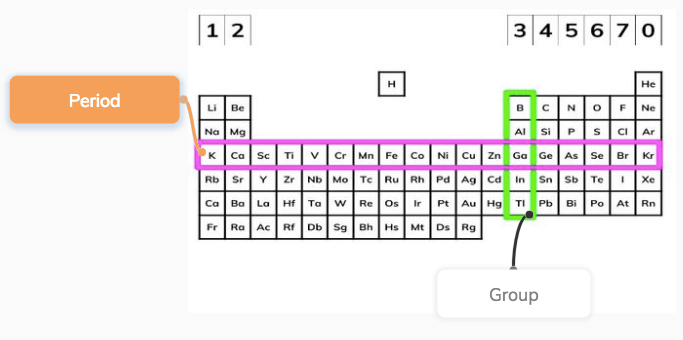
Periods
The table is ‘periodic’ because elements with similar properties occur at regular intervals, i.e. periodically. Therefore, the rows of the periodic table are called periods.
Groups
Elements in the same column have the same number of electrons in their outer shell (the highest energy level). Columns are called groups, and the group number equals the number of electrons an atom of that element has in its outer shell.
Predicting chemical properties
Because all elements in a column have the same number of electrons in their outer shell, they have similar chemical properties. This means they will all react in similar ways.
Want to learn more about Periodic Table?
Join Seneca to get 250+ free exam board specfic A Level, GCSE, KS3 & KS2 online courses.





