Atom Size & Number
Atoms and Sub-Atomic Particles
Atoms are tiny and very light. They are made up of sub-atomic particles (protons, neutrons, and electrons), which are even smaller and lighter than an atom.
**Size: **Atoms have an average radius of about 0.1 nm. About 5 million hydrogen atoms could fit into a pin head. The nucleus of an atom is 10,000 times smaller than the atom. If an atom was the size of Wembley Stadium, then the nucleus would be the size of a garden pea.
**Mass: **Sub-atomic particles: Relative mass of protons and neutrons = 1. Relative mass of electrons = 0.0005 (this gets rounded to zero).
Atoms: Relative mass = number of protons + number of neutrons.
Atomic Number and Mass Number
The mass number of an atom (also called relative mass) is the total number of protons and neutrons in a nucleus. The atomic number of an atom is the number of protons in the atom. This information can be found for each element in the periodic table.
Sodium: Mass number (top) = 23. Atomic number (bottom) = 11.
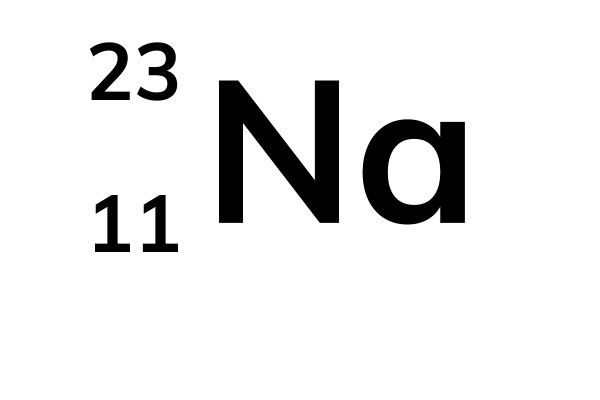
Want to learn more about Atom Size & Number?
Join Seneca to get 250+ free exam board specfic A Level, GCSE, KS3 & KS2 online courses.





